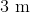All AP Physics 2 Resources
Example Questions
Example Question #9 : Principles Of Quantum Mechanics
Two grams of helium are completely converted into energy and used to power a 
The energy from the two grams of helium can be found using
This energy can then be equated to the man's kinetic energy, which can then be used to find the man's velocity.
Example Question #1 : Principles Of Quantum Mechanics
If the combination of protons and neutrons in an atom's nucleus results in a mass defect of 
In this question, we're given the mass defect of an atom's nucleus and are asked to find the binding energy for this atom.
To begin with, it's important to understand that when protons and neutrons come to be held together within the nucleus of an atom, there is a tremendously powerful force holding them together. This incredibly large force accounts for the mass defect. In other words, the total mass of the nucleus is smaller than the sum of the individual masses of the protons and neutrons that make up that nucleus, and this is due to the strong force.
Einstein's mass-energy equivalence explains the observable mass defect; the mass lost is converted into an enormous amount of energy according to the following equation.
But first, we'll need to convert the mass given to us in the question stem into grams.
Furthermore, because we know the value for the speed of light, we can use this information to solve for the binding energy.
Example Question #341 : Ap Physics 2
A student on the Earth observes a spaceship moving at a considerable fraction of the speed of light. As a result of the spaceship's motion, the student observes that the clocks aboard the spaceship are running slower than when the spaceship was on the Earth. When a student aboard the spaceship observes the student's clock on the Earth, what effect will he notice on the rate of the Earth-bound clock?
The Earth-bound clock appears to run faster
The Earth-bound clock runs at the same rate
The Earth-bound clock appears to run slower
The Earth-bound clock first runs fast, then slow
The Earth-bound clock first runs slow, then fast
The Earth-bound clock appears to run slower
For the observer on the spaceship, the Earth appears to be moving, and special relativity tells us that any observer moving at constant speed can consider him/herself stopped. Therefore, the clock on the Earth experiences the same slowing from the spaceship's viewpoint as the spaceship's clock from the Earth viewpoint. It's a little bit like looking at someone from far away. They look small due to the distance, but you do not look big to them, you also look smaller.
Example Question #342 : Ap Physics 2
Determine the observed length of a 

Using the formula for length contraction:
Where 



Plugging in values
Example Question #343 : Ap Physics 2
Determine the observed length of a 

None of these
Use the following equation:
Where 



Plugging in values
Example Question #11 : Quantum And Nuclear Physics
A 

None of these
Using
Plugging in values
Example Question #345 : Ap Physics 2
A 

None of these
Using
Plugging in values
Example Question #1 : Other Principles Of Quantum Mechanics
A proton is confined to a one-dimensional box of length 



Not enough information to solve the problem.
Second excited state.
Ground state.
Fourth excited state.
Second excited state.
The energy of the quantum system in the 
where 


where 



Since the ground state is 
Example Question #342 : Ap Physics 2
The expectation value 
If the particle exists or not
The momentum of the particle
The exact location of the particle
The energy of the particle
The most probable location of the particle
The most probable location of the particle
From a statistical standpoint, the expectation value of the position, 
Example Question #343 : Ap Physics 2
If a particle in a quantum system is bounded, this means what about the calculated particle energy?
The energy of the particle can be anything, positive or negative.
The energy must be negative and continuous.
The energy must be positive and continuous.
The energy is described by discrete energy levels, and the particle can only have energy that corresponds to these levels, positive or negative.
There is nothing constraining the energy of the particle.
The energy is described by discrete energy levels, and the particle can only have energy that corresponds to these levels, positive or negative.
A key characteristic of bound-state systems is the quantization of the energy into discrete energy levels. The energy can be negative or positive, but the particle can only have an energy that corresponds to one of these energy levels, nothing other. An example is the energy of a hydrogen atom, where the energy levels are given by:
These discrete numbers come from the energy of an electron, which is the fundamental charge.
Certified Tutor
Certified Tutor
All AP Physics 2 Resources