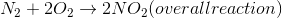All MCAT Physical Resources
Example Questions
Example Question #1073 : Mcat Physical Sciences
The formation of nitrous oxide is a 2-step process.
The overall enthalpy of the reaction is +68kJ.
Given the above information, which of the following statements must be true?
The forward overall reaction has a higher activation energy than the reverse overall reaction.
Both of the steps are exothermic.
Both of the steps are endothermic.
The overall reaction releases heat to the surroundings.
The forward overall reaction has a higher activation energy than the reverse overall reaction.
When a reaction has a positive enthalpy change, it is said to be endothermic. This means that it requires an addition of heat into the system. Because the forward reaction requires more energy than the reverse reaction in order to take place, we see that the activation energy of the endothermic reaction is greater than the activation energy of the reverse reaction.
Essentially, the forward reaction requires a net input of energy, while the reverse reaction results in a net release of energy.
Example Question #1081 : Mcat Physical Sciences
Which of the following is true in the reaction above?
The process of bond formation in chemistry is exothermic. By definition, exothermic reactions have a negative enthalpy change; therefore 
Reducing moles of gas will decrease entropy, meaning that 
Example Question #1081 : Mcat Physical Sciences
Based on the energy diagram, which chemical processes are exothermic?

An exothermic process, by definition, involves a reaction in which the products are lower in energy than the reactants. The reduction in chemical energy results in a release of heat from the reaction.
In the diagram, the path between points is irrelevant. We are simply looking for any instances in which the product point is below the reactant point. Point C has less energy than point B, and point D has less energy than A, B, or C. Transitions from B to C, C to D, or A to D will all result in a reduction of chemical energy, and a release of heat.
Example Question #6 : Endothermic And Exothermic Reactions
Electronegativity is an important concept in physical chemistry, and often used to help quantify the dipole moment of polar compounds. Polar compounds are different from those compounds that are purely nonpolar or purely ionic. An example can be seen by contrasting sodium chloride, NaCl, with an organic molecule, R-C-OH. The former is purely ionic, and the latter is polar covalent.
When comparing more than one polar covalent molecule, we use the dipole moment value to help us determine relative strength of polarity. Dipole moment, however, is dependent on the electronegativity of the atoms making up the bond. Electronegativity is a property inherent to the atom in question, whereas dipole moment is a property of the bond between them.
For example, oxygen has an electronegativity of 3.44, and hydrogen of 2.20. In other words, oxygen more strongly attracts electrons when in a bond with hydrogen. This leads to the O-H bond having a dipole moment.
When all the dipole moments of polar bonds in a molecule are summed, the molecular dipole moment results, as per the following equation.
Dipole moment = charge * separation distance
A scientist decides that the polar nature of water means that it will be useful to help him investigate the solution chemistry of a salt. He dissolves the salt in water, and is surprised to find that the beaker becomes very hot to the touch. Which of the following is likely true of this reaction?
Assume the system is defined as the beaker with the solute and solvent.
More energy is released when hydrated ions form than is absorbed when ionic bonds break
Less energy is released when hydrated ions form than is absorbed when ionic bonds break
Equal energy is released when hydrated ions form than is absorbed when ionic bonds break
Entropy of the system must increase
The reaction is strongly endothermic
More energy is released when hydrated ions form than is absorbed when ionic bonds break
Energy is always released when bonds form, and always absorbed when bonds break. In the situation described in the question, heat is released when the salt dissolves because energy is released when the water forms dipole-based bonds with the newly dissolved ions. This energy is larger in magnitude than the energy that is absorbed to break the ionic bonds in the salt, thus, there is a net flow of heat out of the system.
The entropy of the system must not necessarily increase in this case, because the entropy of the universe is increasing owing to the release of heat. This could be enough to offset a local decrease in entropy in the system.
Example Question #1 : Endothermic And Exothermic Reactions
5.6g of manganese reacts with 650mL of 6.0M hydrochloric acid to form manganese (V) chloride and hydrogen gas. Along with the products, a large amount of heat is evolved.
This reaction is classified as __________.
Exothermic, with a negative
Endothermic, with a positive
Exothermic, with a positive
Endothermic, with a negative
Exothermic, with a negative
Any reaction in which heat is evolved (released) is classified as exothermic. The change in heat content (
Example Question #8 : Endothermic And Exothermic Reactions
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In scenario 2, the reaction that water undergoes would best be characterized as __________.
endothermic in this scenario, and spontaneous at standard conditions
endothermic in this scenario, and nonspontaneous at standard conditions
exothermic in this scenario, and nonspontaneous at standard conditions
exothermic in this scenario, and spontaneous at standard conditions
exothermic in this scenario, and nonspontaneous at current conditions
endothermic in this scenario, and nonspontaneous at standard conditions
The "reaction that water undergoes" is melting. Melting is endothermic, as the reaction written in the pre-question passage illustrates. Standard conditions are defined as 0C and 1 atm of pressure. Water freezes at 0C, and thus the melting reaction is nonspontaneous at these conditions.
Example Question #9 : Endothermic And Exothermic Reactions
A system experiences the following energetic changes:
Which of the following is true?
The system loses 
The system loses heat



Remember that a negative sign denotes an exothermic process (heat is released into the surroundings), while a positive energy change denotes an endothermic process (heat is absorbed).
Using the given values we can calculate the total energy change of the system:
Since the overall energy change is positive, 
Example Question #61 : Thermochemistry And Energetics
Is the reaction above endothermic or exothermic?
Endothermic because an increase in temperture would cause the reaction to shift to the left
Exothermic because an increase in temperture would cause the reaction to shift to the right
Exothermic because an increase in temperture would cause the reaction to shift to the left
Endothermic because an increase in temperture would cause the reaction to shift to the right
Exothermic because an increase in temperture would cause the reaction to shift to the left
The reaction is an exothermic reaction, since the heat is added on the products side. An endothermic reaction would have the heat on the reactants side. An increase in heat in this reaction would cause the Keq to decrease, and there will be a shift to the left of the reaction (toward the reactants).
Remember that Keq is dependent on temperature, and can be affected by changes in heat. Keep in mind, also, that the reverse reaction that occurs during the leftward shift will be endothermic, using the additional heat as a reactant rather than a product.
Example Question #1081 : Mcat Physical Sciences
A student is performing a reaction with unknown compounds in his chemistry lab. The only information the student knows about the reaction is that it is endothermic and reversible. Using this knowledge alone, how can the student increase the yield of his product?
Decrease the temperature
Add product to the reaction
Remove reactant from the reaction
Increase the temperature
Increase the temperature
To answer this question, we need to have a solid understanding of Le Chatelier's principle.
In an endothermic reaction, heat is needed to facilitate the reaction. To increase the products, we want to shift the reaction to the right.
We should already know that adding product or removing reactant shifts the equilibrium to the left, and yields more starting material rather than product. In an endothermic reaction, we can consider heat as a reactant; thus, adding heat (increasing temperature) would allow us to shift the reaction to the right.
Decreasing the temperature, removing reactant, or adding product would all increase the yield of the starting materials.
Example Question #1082 : Mcat Physical Sciences
For any given chemical reaction, one can draw an energy diagram. Energy diagrams depict the energy levels of the different steps in a reaction, while also indicating the net change in energy and giving clues to relative reaction rate.
Below, a reaction diagram is shown for a reaction that a scientist is studying in a lab. A student began the reaction the evening before, but the scientist is unsure as to the type of the reaction. He cannot find the student’s notes, except for the reaction diagram below.

Growing frustrated by his inability to decipher what chemical reaction the student had started, the scientist decides to measure the reaction vessel's temperature. Based only on the above reaction diagram, what is he most likely to find if the reaction is ongoing?
(Assume that the reaction vessel is defined as the system, and that the entropy of the system decreases)
The products of this reaction have an equal energy level to the reactants
A cold container from an endothermic reaction
A warm container from an endothermic reaction
A cold container from an exothermic reaction
A warm container from an exothermic reaction
A warm container from an exothermic reaction
Point 5 is the energy level of the products of the reaction, while point 1 is the energy level of the reactants. Point 5 is lower than is point 1, indicating that the products of this reaction contain lower overall energy than do the reactants. This energy must be released in some form, likely as heat, characteristic of an exothermic reaction.
The question further specifies that there is a local decrease in entropy of the system, thus, the only way that entropy of the universe can increase is to release heat and increase the entropy of the surroundings.
Certified Tutor
Certified Tutor
All MCAT Physical Resources