All MCAT Physical Resources
Example Questions
Example Question #3 : Laws Of Thermodynamics
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
The same scientist in the passage measures the variables of another reaction in the lab. He knows that this reaction is spontaneous under standard conditions, with a standard free energy change of –43 kJ/mol. Using laboratory-calculated variables, he determines that the Gibbs Free Energy has a value of 0 kJ/mol. What can we say about this reaction?
The reaction had reached equilibrium when the scientist made his measurements.
It indicates that the reaction will produce 43 kJ of energy for every mole that is allowed to react spontaneously under current conditions.
43 kJ of energy must be put into the system to have the reaction proceed spontaneously.
The scientist made an error, all spontaneous reactions have a negative Gibbs Free Energy value.
The reaction will always produce 43 kJ of heat when it reacts to completion.
The reaction had reached equilibrium when the scientist made his measurements.
A reaction with a zero free energy change is at equilibrium. At standard conditions, not at equilibrium, this reaction would have a Gibbs free energy change of –43 kJ/mol, would be spontaneous, and would be able to produce 43 kJ of useful work for every mole that reacts.
Example Question #21 : Thermochemistry And Energetics
An air conditioning unit is supplied with power to effectively cool an entire house without generating heat.
Which law(s) of thermodynamics is(are) violated?
I. The first law
II. The second law
III. The third law
II only
II and III
III only
I and II
I only
II only
The first law of thermodynamics, conservation of energy, is not violated since the refrigerator is merely transforming electrical energy into work.
The second law of thermodynamics, increase of entropy, is violated since the entropy of a cooled house is lower, but there is no corresponding increase in entropy elsewhere. In order to cool an object in the system, and object in the surroundings must be heated.
The third law of thermodynamics deals with absolute zero and is not relevant.
Example Question #40 : Physical Chemistry
Consider the following reaction.
Which of the following is true about this reaction?
This reaction is exothermic because the free energy change is less than zero
This reaction increases the entropy of the universe because it is a spontaneous reaction
This reaction is endergonic because the enthalpy change is greater than zero
This reaction increases the entropy of the universe because it is a non-spontaneous reaction
This reaction increases the entropy of the universe because it is a spontaneous reaction
The question states that the change in Gibbs free energy, 
This reaction is exergonic (spontaneous) because the change in Gibbs free energy (

Example Question #1051 : Mcat Physical Sciences
Which of the following will result in heat transfer?
Entropy increases
A temperature gradient exists
Internal energy is stable
Enthalpy increases
A temperature gradient exists
Heat is a form of energy resulting from molecular velocity. Temperature is a scale to measure the amount of internal energy stored as heat, known as enthalpy. Entropy is the relative disorder of a system.
In order for a heat transfer to occur, the system must not be in equilibrium. In other words, there must be a difference in heat energy between two regions of the system, which will allow heat energy to travel from the region of high energy to the region of low energy. This condition is easily identified by a temperature gradient, in which heat will from the region of high temperature to the region of low temperature until the system is at an equilibrium temperature.
Example Question #1 : Laws Of Thermodynamics
Which of the following is an implication of the zeroth law of thermodynamics?
If systems A and B are in thermal equilibrium with system C, then systems A, B, and C are in thermal equilibrium with each other
Energy in the universe cannot be created or destroyed
A pure crystal kept at 
Entropy of the universe is always increasing
If systems A and B are in thermal equilibrium with system C, then systems A, B, and C are in thermal equilibrium with each other
This question asks about the zeroth law of thermodynamics which states if two systems are in thermal equilibrium with another system, then all three systems will be in thermal equilibrium with each other.
The other answer choices in this question talk about the other three laws of thermodynamics. First law of thermodynamics states that energy of the universe is constant and that energy cannot be created or destroyed. The second law states that the entropy of the universe is constantly increasing, even if local entropy decreases. The third law states that a pure crystal at absolute zero 
Example Question #43 : Physical Chemistry
Two children are playing on an icy lake. Child 1 weighs 50kg, and child 2 weighs 38kg. Child 1 has a backpack that weighs 10kg, and child 2 has a backpack that weighs 5kg.
Over the course of the afternoon, they collide many times. Four collisions are described below.
Collision 1:
Child 1 starts from the top of a ramp, and after going down, reaches the lake surface while going 5m/s and subsequently slides into a stationary child 2. They remain linked together after the collision.
Collision 2:
Child 1 and child 2 are sliding in the same direction. Child 2, moving at 10m/s, slides into child 1, moving at 2m/s.
Collision 3:
The two children collide while traveling in opposite directions at 10m/s each.
Collision 4:
The two children push off from one another’s back, and begin moving in exactly opposite directions. Child 2 moves with a velocity of +8m/s.
In collision 1, child 1 stops moving under the opposing force of friction. After she stops moving, the energy she lost to the lake as heat, via friction, is exactly equal to the amount of kinetic energy she lost from the moment she struck the lake surface to when she stopped. What law prevents this lost heat energy from ever moving the child again?
Second Law of Thermodynamics
First Law of Thermodynamics
Zeroth Law of Thermodynamics
Third Law of Thermodynamics
The principle that entropy of the universe always decreases
Second Law of Thermodynamics
The Second Law of Thermodynamics states that entropy of the universe always increases. The loss of heat energy to the lake due to friction leads to an increase in the entropy of the universe, and thus the return of that energy to move the child would lead to a decrease in the entropy of the universe.
Nevertheless, the hypothetical movement of the child with this energy is allowed by the First Law of Thermodynamics.
Example Question #43 : Physical Chemistry
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
The cup of water is the system, and the snow is the surroundings.
The cup of water is the system, and the stove is the surroundings.
The cup of water is the system, and the snow, stove, and remainder of the universe are the surroundings.
The cup of water, snow, and stove comprise the system; the universe is the surroundings
The system is all of the universe, except the stove used to input energy.
The cup of water is the system, and the snow, stove, and remainder of the universe are the surroundings.
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
Example Question #44 : Physical Chemistry
The combustion of propane is given by the following formula.
If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
1477.9kJ
641.95kJ
–1477.9kJ
–641.95kJ
–1477.9kJ
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.
Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
Example Question #1051 : Mcat Physical Sciences
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




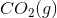


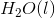

Calculate the 
To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
Example Question #2 : Thermodynamic Systems And Calorimetry
How much energy must a stove transfer to completely transform 

To turn the water into steam, the stove must first raise the temperature of the water, and then provide energy to change the phase. This requires two distinct steps.
Find the energy to raise the temperature of the water using the equation 
Now, we need to find the energy needed to convert the water to a gas. We will use the equation 
Finally, we add the energy for the two steps to find the total energy required.
Certified Tutor
Certified Tutor
All MCAT Physical Resources


































