All MCAT Physical Resources
Example Questions
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
The strain on an object __________ when initial length is increased and __________ when final length is increased.
increases . . . decreases
decreases . . . increases
increases . . . increases
decreases . . . decreases
decreases . . . increases
Strain of an object is defined as:




Example Question #12 : Biochemistry, Organic Chemistry, And Other Concepts
Which of the following will result in the greatest increase in length of a rubber band at its maximum stretch point, as compared to an unaltered control?
Assume the rubber band will experience the same constant force.
Using a rubber band that has twice the cross-sectional area
Using a rubber band that has half the length
Using a rubber band that is twice as elastic
Using a rubber band that is twice as long and is twice as elastic
Using a rubber band that is twice as elastic
To answer this question, we need to know the equation for Young’s modulus, 
Recall the definitions of stress and strain:
Here, 



Rearranging this equation to solve for change in length gives us:
Since we want to increase 





Using half the length (
Example Question #13 : Biochemistry, Organic Chemistry, And Other Concepts
Which of the following is false regarding Young’s modulus?
Young’s modulus is defined as the slope of the stress vs. strain plot
Young’s modulus is only valid for certain stresses
Young’s modulus increases as stress increases
Young’s modulus has the same units as stress
Young’s modulus increases as stress increases
Young’s modulus is a constant that is calculated by generating a stress vs. strain plot. The slope of the linear region of the stress vs. strain plot is defined as the Young’s modulus. Recall that the stress vs. strain plot is nonlinear past the elastic limit (stress at which the material starts to plastically deform). This means that we cannot calculate the slope past the elastic limit; Young’s modulus is invalid for stresses beyond the elastic limit.
Young’s modulus is defined as follows:
To calculate strain, you divide change in length by initial length. Since both change in length and initial length have the same units, the units cancel and strain becomes a dimensionless quantity. This means that the Young’s modulus will have the same units as stress alone.
Young’s modulus is a material property. This means that the Young’s modulus is constant for a given type of material. It does not depend on the stress experienced by the material. Any changes to stress will be compensated for by a change in strain, which will allow Young’s modulus to be constant for the material. Similarly, Young’s modulus does not depend on strain or on the geometrical properties (length and area) of the material. It will only change if you change the type of material being used.
Example Question #1012 : Mcat Physical Sciences
An IR spectroscopy reading of a compound shows a large absorption at 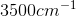
Alcohol
Amide
Amine
Aldehyde
Alcohol
Compounds with an alcohol group show absorptions in an IR sprectrum from 








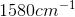



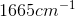
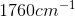
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Consider the following solutions.
Solution A: 1M sodium chloride solution
Solution B: 1M calcium nitrate solution
Solution C: 1M sucrose solution
Equal volumes of the solutions are combined and the mixture is added to a distillation column. Which of the following solutions will separate first?
Solution A
Solution C
These solutions cannot be separated via distillation
Solution B
Solution C
Distillation is a process of separating a liquid from solutes or other liquids. It utilizes the boiling point differences to separate substances. A substance with a low boiling point will evaporate first in a distillation column and will be isolated first. The question is asking which solution will be isolated first; therefore, we need to figure out which solution has the lowest boiling point. Recall that the boiling point of a solution is elevated when there are more solutes present in the solution. Sodium chloride (

Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Small differences in boiling point require the use of __________ distillation and large differences in boiling point require the use of __________ distillation.
simple . . . simple
fractional . . . simple
simple . . . fractional
fractional . . . fractional
fractional . . . simple
There are two types of distillation. Simple distillation is used to separate molecules that have very different boiling points. Fractional distillation is used to separate molecules with small differences in boiling points. Fractional distillation is often used if the difference between boiling points is less than 
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Which of the following mixtures can be separated using fractional distillation (boiling points of each substance given in 
I. Chloroform (62.2) and 
II. Iodine (184.3) and mercury (356.9)
III. Nitric acid (120) and sulfuric acid (310)
I, II, and III
I only
II and III
II only
I, II, and III
Distillation is used to separate molecules with different boiling points. Simple distillation is used to separate molecules with vastly different boiling points. Fractional distillation, on the other hand, is a refined form of simple distillation that can be used to separate molecules with similar boiling points. Note that fractional distillation can separate molecules with either different or similar boiling points; therefore, fractional distillation can be used to separate any of the given mixtures.
Example Question #1 : Distillation
Which of the following conditions will result in the greatest increase in the rate of distillation of a substance?
Decreasing the atmospheric pressure
Decreasing the temperature
Decreasing the mole fraction of the substance
Decreasing the vapor pressure
Decreasing the atmospheric pressure
Rate of distillation is increased when the ability of a substance to become a vapor is increased. Recall that vapor is created when enough heat is applied to the liquid. The temperature at which the liquid becomes vapor is called the boiling point. A liquid turns into a vapor when the vapor pressure (pressure applied by the vapor from the liquid) equals the atmospheric pressure. Decreasing the atmospheric pressure will make it easier for the liquid to turn into a vapor; therefore, this will increase the rate of distillation.
Decreasing the vapor pressure will remove vapor from system. This will make it harder to distill substances. Decreasing temperature will move the system away from the boiling point, thereby decreasing the amount of vapor. Decreasing mole fraction of the substance will decrease the surface area of the substance (at the surface of the solution). Liquid molecules need to be present at the surface to escape the solution and become vapor; therefore, decreasing mole fraction will decrease the amount of vapor.
Example Question #1 : Thin Layer Chromatography
A new student is planning to use thin layer chromatography (TLC) for his research project. After setting up the apparatus the student forgets to place a lid on the TLC jar. He obtains poor results after running the TLC experiment. Which of the following can best explain his bad results?
The open system evaporates the solvent in the solution
The open system evaporates the solvent on the TLC plate
The open system prevents the evaporation of the solvent on the TLC plate
The open system prevents the evaporation of the solvent in the solution
The open system evaporates the solvent on the TLC plate
TLC is a laboratory technique commonly used to separate components of a mixture. Mixtures are placed on the TLC plate (stationary phase), which is then transferred to a jar containing the solvent. The solvent travels through the plate and carries components of the mixture along with it. Based on its properties, each component is dragged to different distances on the plate. The relative distances travelled by each component can be used for separation and identification.
It is important to place a lid on the jar because the solvent will be a volatile substance. An open system will allow for the solvent to evaporate from the TLC plate and reduce the amount of solvent travelling through the plate. The solvent in the solution will evaporate, but it is negligible and inconsequential to the data collected on the TLC plate.
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Which of the following can be determined from thin layer chromatography results?
I. Number of components in the mixture
II. The identity of the components
III. Polarity of components
II and III
I, II, and III
II only
I and III
I and III
Thin layer chromatography (TLC) is used to separate components in a mixture. Components are separated on a TLC plate because each component travels a different distance. The distance travelled depends on several factors. One of those factors is polarity; therefore, TLC can used to determine polarity of substances.
TLC is not useful for identifying substances. Other techniques such as NMR and IR spectroscopy are useful for identifying individual components. The Rf value of a compound can help identify it in a broad sense, but this technique provides only very rudimentary identification capabilities.
Certified Tutor
All MCAT Physical Resources










