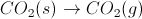All MCAT Physical Resources
Example Questions
Example Question #1 : Enthalpy
The formation of nitrous oxide is a 2-step process.
The overall enthalpy of the reaction is +68kJ.
If the enthalpy change of step 1 is 180kJ, what is the enthalpy change of step 2?
248kJ
–112kJ
More information is needed to answer the question.
112kJ
–112kJ
Hess's law states that the enthalpies of the steps in an overall reaction can be added in order to find the overall enthalpy. Since the overall enthalpy of the reaction is +68kJ, we can use the following equation to find step 2's enthalpy.
Example Question #2 : Enthalpy
Consider the following processes:
1)
2)
3)
Which for the following expresses 

1)
2)
3)
We need to add the given processes in such a way that leaves 

Since there are no 
First, reverse process 2 and multiply by two:
2a)
2a)
2a)
Add this result to process 1.
1)
2a)
1+2a)
Now we need to combine with process 3 to eliminate the 
3a)
3a)
Add this to the combined result from processes 1 and 2.
1+2a)
3a)
1+2a+3a)
This gives our final combination. The elemental reaction is 

Example Question #41 : Thermochemistry And Energetics
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
After the processes in each of the four scenarios above, what can be said about the entropy of the universe?
It increases in scenarios 2 and 4, only.
It increases in all scenarios.
It increases in scenarios 3 and 4, only.
It increases in scenarios 1 and 2, only.
It increases in scenarios 1 and 3, only.
It increases in scenarios 1 and 2, only.
Scenarios 3 and 4 are presented as thought experiments because, common sense tells us, they will never happen. The fundamental reason they will never happen is because they do not lead to a total increase in entropy of the universe. In order for an event to proceed, this requirement must be satisfied.
Example Question #42 : Thermochemistry And Energetics
Which of the following chemical or physical processes involves an increase in entropy?
Picking up a set of 
Entropy describes the number of configurations a system can have or, alternatively, how much "order" it possesses.
When water freezes, it forms a crystal lattice, which is more ordered than liquid water. Likewise, collecting playing cards and stacking them in a deck involves a transformation from disorder (cards scattered around the floor) to order (a single deck). Chemically, the precipitation of 



The disproportionation of 

Example Question #1 : Entropy
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
A scientist studying the dissociation of the Arrhenius base sodium hydroxide discovers that the reaction is very exothermic. What is true of the entropy change in the surroundings?
Assume the system refers to the reaction vesselcontaining aqueous sodium hydroxide.
The entropy of the surroundings is zero
The entropy of the surroundings must remain constant
The entropy of the surroundings decreases
The entropy of the surroundings increases
The entropy of the surroundings may increase or decrease
The entropy of the surroundings increases
The entropy change of the surroundings for an exothermic system must be positive. The release of heat by the exothermic reaction drives the increase in disorder of the environment surrounding the reaction vessel.
As the heat leaves the reaction system, it increases the entropy of the particles in the surroundings.
Example Question #43 : Thermochemistry And Energetics
Which of the following chemical processes involves a decrease in entropy?
Entropy decreases when the number of moles of gas decreases during a reaction. In the case of the correct answer, the number of moles of gas decreases from two to one.
When a substance goes from a solid to a gas (sublimation) or from a liquid to a gas (evaporation), entropy increases. Likewise, when a solid dissolves in water, entropy increases. Entropy (i.e. the number of arrangements a system can have) is much greater in a gas than in a liquid or solid. It is also greater for ions solvated in solution than for an un-dissolved solid.
Note that, in general, the entropy of the universe increases. In order for a process to involve a decrease in entropy of the system, there is likely a consequent increase in entropy of the universe.
Example Question #71 : Biochemistry, Organic Chemistry, And Other Concepts
Which of the following processes involves an increase in entropy?
Crystallization
More than one of the other options
Condensation
Sublimation
Freezing
Sublimation
Entropy is a measure of disorder or randomness. A system with more random motion between molecules has greater entropy. The phases of matter in order of increasing entropy are solid, liquid, then gas. The processes that increase entropy by changing phases will cause a phase transition from lower entropy to higher entropy. These transitions are melting (solid to liquid), vaporization (liquid to gas), and sublimation (solid to gas).
Example Question #1 : Entropy
Which of the following does not represent an increase in entropy?
A system's temperature increases by
An increase in entropy will result when a solid is dissolved into a liquid because disorder is increased due to the presence of a greater number of particulates (ions). Similarly, any reaction that results in more molecules represents an increase in entropy, as in the example of the decomposition of hydrogen peroxide. A temperature increase does not relate to entropy; it only helps describe the energy of the reaction. An increase in temperature usually signifies an endothermic process, but does not give an indication about entropy.
The only correct answer is the chemical reaction between aluminum and iron (III) oxide, which produces an equal amount of particles and does not involve any phase change.
Example Question #1071 : Mcat Physical Sciences
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


A scientist is creating a solution to study vapor pressure. When she adds the solute, intermolecular bonds in the solute break to allow the solution to form. The formation of the solution is exothermic. Which of the following is true?
Forming solute-solvent bonds absorbs energy
Breaking solute-solute bonds releases energy
Breaking solute-solute bonds absorbs energy
Forming solute-solute bonds absorbs energy
Breaking solvent-solvent bonds releases energy
Breaking solute-solute bonds absorbs energy
Even though the dissolution process is exothermic, breaking bonds ALWAYS requires energy input. In the case of an exothermic dissolution, more energy is released when solute-solvent bonds are formed, than was absorbed when solute-solute bonds were broken. The initial step requires an input of activation energy, but the final product allows for a net energy release.
Example Question #61 : Physical Chemistry
When a solution is formed, which of the following is true?
The bonds between the solvent molecules are broken in an exothermic process
Formation of a solution causes a decrease in entropy
Solution formation that is exothermic results in stronger intermolecular bonds, compared to the bonds in the pure substances
The bonds in the solute molecules are broken in an exothermic process
None of these statements are true
Solution formation that is exothermic results in stronger intermolecular bonds, compared to the bonds in the pure substances
When dealing with the enthalpy of a solution, there are three specific steps, each having their own enthalpy.
1) The breaking of solute-solute
2) The breaking of solvent-solvent bonds
3) The formation of solute-solvent bonds
Remember that breaking bonds always requires an input of energy, and is thus considered endothermic. The formation of new bonds is an exothermic process. When an overall solution is exothermic, it means that the new intermolecular bonds are more stable. A system with less energy is considered more stable.
Certified Tutor
Certified Tutor
All MCAT Physical Resources