All Organic Chemistry Resources
Example Questions
Example Question #2 : Using Pcc
What is the product of 1-pentanol when it is treated with PCC?
1-pentanone
Pentanal
None of these
2-pentanol
No reaction occurs
Pentanal
PCC is an oxidizing agent. It converts alcohols to carbonyls, but is not strong enough to convert a primary alcohol into a carboxylic acid. It only converts primary alcohols to aldehydes, and secondary alcohols to ketones. 1-pentanol is a primary alcohol so it will be converted to the aldehyde pentanal.
Example Question #3 : Using Pcc

Which reagent is best-suited to accomplish the given reaction?
PCC
PCC
PCC is an oxidizing agent that reacts with primary and secondary alcohols. However, it is less reactive than potassium permanganate and chromic acid. PCC differs from chromic acid by oxidizing primary alcohols to aldehydes, whereas chromic acid oxidizes primary alcohols and aldehydes to carboxylic acids. The desired product of the reaction given requires that the primary alcohol be oxidized to an aldehyde, so PCC is the best option. 
Example Question #1 : Using Pcc
Which reagents are required to drive the given reaction?
This is a two step reaction. In the first step, an alcohol is substituted for the bromine via an 

Example Question #1 : Organic Oxidizing Agents
Which of the following is not true regarding the reagent 

None of these





Example Question #1 : Using Pcc
What is the product of the reaction shown?


I
IV
II
V
III
III
First step: PCC oxidizes the primary alcohol to acetaldehyde
Second step: Grignard reagent attacks carbonyl carbon
Third step: Neutralization of the anion forms isoproyl alcohol
Example Question #452 : Organic Chemistry
Which of the following compounds is not a reducing agent?

Example Question #7 : Redox Chemistry

What would be the product of the given reaction?





The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in 

Example Question #10 : Organic Oxidizing Agents

What would be the product of the given reaction?





The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in 

Example Question #11 : Redox Chemistry
What is the product of the reaction shown?
PCC can be used to oxidize primary alcohols into aldehydes, or secondary alcohols into ketones. The starting material shown is a secondary alcohol, so the product will be a ketone (a carbonyl (
Example Question #12 : Redox Chemistry
A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange?
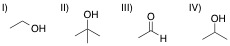
IV
I
All of the solutions will lose their orange color, indicating oxidation
III
II
II
The following reaction schemes show the oxidation of all substrates, indicating that substrate II is in the highest oxidation state possible, and that an oxidation of this compound will not proceed.
Remember that in sulfuric acid and water, Na2Cr2O7 will be converted to CrO3, the active oxidant species. Furthermore, the oxidation mechanism involving this species includes the key step in which a hydrogen bonded to the carbon in question is eliminated, and simutaneously, a double bond from that carbon to an oxygen is installed. Thus, all substrates that feature at least one hydrogen bonded to the carbon to be oxidized can and will be oxidized in the precense of chromium trioxide.
Lastly, remember that these reactions are taking place in the prescence of water. While substrates such as compound III do not appear to be oxidizable, attack of water at the aldehyde carbon will give a dialcohol tetrathedral intermediate that can be immediately oxidized by chromium trioxide to the corresponding carboxylic acid. A similar mechanism occurs for substrate I, wherein, after the ketone oxidation state is achieved, an attack of water furnishes the same dialcohol intermediate that is oxidized to the carboxylic acid. Remember that the highest oxidation state available for organic compounds containing more than one carbon is the carboxylic acid oxidation state. Chromium trioxide will oxidize all organics to this oxidation state, unless directly-bonded hydrogens are not present in lower oxidation states, such as shown with substrate IV.

Certified Tutor
All Organic Chemistry Resources



























