All Organic Chemistry Resources
Example Questions
Example Question #2 : Oxidation Reduction Reactions
A water molecule is converted to hydrogen peroxide through a series of reactions. What can you conclude about the oxygen molecule?
Oxygen is reduced because it gains an electron
Oxygen is oxidized because it loses an electron
Oxygen is oxidized because it gains an electron
Oxygen is reduced because it loses an electron
Oxygen is oxidized because it loses an electron
Water, 



Example Question #2 : Oxidation Reduction Reactions
Which of the following is true regarding oxidation?
I. Oxidation increases the oxidation number
II. Oxidizing agent is always reduced
III. Oxidation of an atom is always spontaneous
I and III
I and II
III only
II and III
I and II
Oxidation is the process of removing electrons from an atom. This increases the oxidation number (makes oxidation number more positive). An atom undergoes oxidation by losing electrons and donating them to another atom. Since it is facilitating reduction (gain of electrons) of another atom, an atom that is oxidized is also called a reducing agent. Oxidizing agents, on the other hand, are reduced (gain electrons) and facilitate the oxidation of other atoms (removal of electrons from other atoms). Oxidation of an atom does not depend on the Gibbs free energy; therefore, oxidation can be spontaneous or nonspontaneous.
Example Question #1 : Help With Reduction
In general, the reduction of a ketone to an alcohol can be accomplished by all of the following except one. Which one will not reduce a ketone?
Hydride nucleophile

Example Question #1 : Help With Reduction
In which of the following reactions is sodium reduced?
Both of these reduce sodium
None of these reduce sodium
Conversion of sodium chloride to sodium bromide
Conversion sodium chloride to sodium sulfide
None of these reduce sodium
Reduction involves gain of electrons whereas oxidation involves loss of electrons. In reduction, the oxidation number becomes more negative (due to gain of electrons) whereas in oxidation, the oxidation number becomes more positive. Sodium is an alkali metal, found on the first column of the periodic table. Every atom in this column has one valence electron; therefore, to complete its octet every alkali metal will lose an electron and will have an oxidation number of 

Example Question #42 : Redox Chemistry
A molecule undergoing oxidation __________ protons and a molecule undergoing reduction __________ protons.
loses . . . does not lose
gains . . . loses
does not lose . . . does not lose
loses . . . gains
does not lose . . . does not lose
Oxidation and reduction involve loss and gain of electrons, respectively. It does not involve loss or gain of protons. Recall that the identity of an atom is changed when the amount of protons change; therefore, it is very hard to change the amount of protons. Only few reactions (such as nuclear decay reactions) can change the number of protons and alter the identity of an atom.
Example Question #1 : Substitution Mechanisms
Which of the following substrates would have the fastest reaction rate for an SN1 mechanism?
The SN1 mechanism involves the formation of a carbocation intermediate in the rate-determining step. The most stable carbocation will produce the fastest reaction. We can immediately eliminate any answer choices that will produce primary or secondary carbocations, since a tertiary carbocation will be much more stable. When comparing tertiary carbocations, larger and more electronegative substituents will allow for more charge stabilization.
Since the tertiary carbocation formed by the dissociation of iodide from 
Example Question #491 : Organic Chemistry
Which of the following determines the general rate of an 
Rate=k[nucleophile]
Rate=k[substrate][base]
Rate=k[substrate][nucleophile]
Rate=k[substrate]
Rate=k[substrate]
The rate of an 


Example Question #3 : Substitution Mechanisms
What is the final product of the pictured reaction?
1.
2.
3.
No reaction
Keep in mind that after the aldehyde is reduced into an alcohol, the molecule can undergo an intramolecular reaction, as alcohol is a good nucleophile and the halogen is a stellar leaving group.
Example Question #1 : Substitution Mechanisms
Which of the following is not true for an SN1 reaction?
All are true
Rearrangements are possible
A strong nucleophile is required
Racemization of products
A strong nucleophile is required
A strong nucleophile is not required for SN1. A weak nucleophile may be used. Remember that the SN1 mechanism goes through a carbocation intermediate.
Rearrangements are possible for SN1 reactions (not SN2). A rearrangement will occur to create a more stable intermediate in the mechanism. For example, if the carbocation is secondary, a methyl shift may occur to make the carbocation intermediate tertiary.
A racemic mixture of products occurs when with the nucleophile may attack the carbocation from either the top face or bottom face. When a reaction goes through a carbocation intermediate, as in SN1, there may be a racemic mix of products.
SN1 is unimolecular, and the rate of the reaction is determined by the substrate and reaction constant.
Example Question #1 : Help With Sn1 Reactions
A student carried out a substitution reaction in the lab using ethanol as a solvent. The student began with an optically pure reactant (100% (R)-configuration) and finished with a racemic mixture of products (50% (R)-configuration, 50% (S)-configuration).
The reaction went through which of the following mechanisms?
SN1
E1
SN2
E2
Either SN1 or SN2
SN1
SN1 reactions result in racemization when the nucleophile has a 50% chance of attacking the carbocation intermediate from the top face, and a 50% chance of attacking from the bottom face. SN1 reactions are favored in polar protic solvents, such as ethanol.
E2 and E1 are incorrect as they are elimination reaction mechanisms, and we are looking for a substitution mechanism. SN2 reactions result in inversion, not racemization. Additionally we know that SN2 is incorrect because SN2 is favored in polar aprotic solvents.
Certified Tutor
All Organic Chemistry Resources









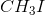
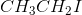








![\small Rate = k[Substrate]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/655607/gif.latex)



