All Organic Chemistry Resources
Example Questions
Example Question #431 : Organic Chemistry
How many stereoisomers are possible for methylcyclopropane?
The correct answer is 2 stereoisomers. To determine the number of stereoisomers that a compound may be present in, the number of stereocenters must be determined. In the case of methylcyclopropane, we have only one stereocenter, which is the carbon in the ring that is bound to the methyl group. Once the number of stereocenters is determined, we can use the following formula to determine the number of stereoisomers:
Where 
Example Question #78 : Stereochemistry
If a given molecule has 5 chiral centers, what is the maximum number of stereoisomers that exist for the molecule.
If a molecule has n sterocenters, it has up to 


Example Question #1 : Help With Meso Compounds
Which of the following are meso compounds?
I. 2,4-dichloropentane
II. 1,3-dimethylcyclopentane
III. 2,3-dichloropentane
I and III
II and III
I only
I and II
II only
I and II
A meso compound has at least two stereocenters, but is not chiral due to an axis of symmetry. Each of the given molecules have two stereocenters. However, if you cut the first molecule in half, you would get two identical half molecules. If you cut the second molecule in half, the same would occur. Thus, I and II have meso stereoisomers. To solve this question, it is easiest to draw out the given molecules.
Example Question #53 : Isomers

Which of the given chair conformations represents a meso compound?
IV
III
I
II
None of these
III
Meso compounds are characterized by an internal plane of symmetry that renders them achiral despite the presence of chiral center(s). For the given six member rings, the key to identifying the meso compound is finding the structure in which the two chlorine atoms are on the same side of the ring. It is also crucial to recognize that six member rings undergo rapid chair-flipping. Identical substituents on the same side of the ring quickly alternate between equatorial and axial positions such that they are on average of the same orientation. Compound III is the only structure given in which the chlorine atoms are facing in the same direction (up in the given conformation). Although one is equatorial and the other is axial, observation of the corresponding Haworth projection (see below) shows that there is indeed an internal plane of symmetry. Thus, compound III is meso.

Example Question #1 : Help With Meso Compounds

Which of these molecules is a meso compound?
I only
III only
IV only
II only
II and III
III only
A molecule is meso if it contains at least two stereocenters, but is rendered optically inactive by internal structural symmetry. In other words, a meso compound may be split in half in some way such that portions on either side of an imaginary line are mirror images. Note: The absolute configurations of a meso compound with two stereocenters are opposite (R/S). The internal symmetry that makes molecule III a meso compound is best conveyed through a Haworth projection:

Example Question #84 : Stereochemistry
How many possible stereoisomers does the product of the following reaction have?


This is the product of the given reaction. Remember, anti elimination is favored over the Zaitsev product. All possible stereoisomers with methyl groups syn are meso compounds. Of the possible stereoisomers with methyl groups anti, there are two pairs of identical structures. Thus there are only 2 possible stereoisomers.
Example Question #61 : Isomers

How many of the existing configurational stereoisomers are chiral?
3
None
1
2
2
There are three configurational stereoisomers. These include the RS, SS, and RR isomers. Since one of them, the RS isomer, has a plane of symmetry, it is achiral, and the other two are chiral.
Example Question #62 : Isomers

How many configurational stereoisomers exist for this structure?
4
2
3
1
3
There are two tetrahedral asymmetrical stereocenters in this molecule (the carbon atoms attached to each of the chlorine atoms). Thus, the combinations of R and S include RR, RS, SR, and SS. Note the plane of symmetry in the molecule; RS and SR are the same molecule (meso compounds). Thus, there are three distinct configurational stereoisomers of this compound.
Example Question #1 : Epimers

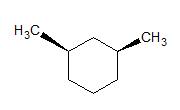
The given molecules are __________.
None of these
stereoisomers
constitutional isomers
identical
conformers
stereoisomers
Stereoisomers have different orientations around a single stereocenter. The two molecules are stereoisomers. Specifically, these molecules are epimers, meaning that they differ at only one stereocenter.
Constitutional isomers have the same molecular formula, but different structures. Conformers have different rotations around a single bond. The molecules are clearly not identical.
Example Question #431 : Organic Chemistry
Which of the following carbons represents the stereogenic center between the given isomers?


Carbon 1
Carbon 2
Carbon 5
Carbon 4
Carbon 3
Carbon 4
Epimers are isomers that have different configurations at only one carbon atom. This carbon atom is known as the stereogenic center. The given compounds are identical except for the orientation around carbon number 4; thus, carbon 4 is the stereogenic center.
Certified Tutor
Certified Tutor
All Organic Chemistry Resources





















