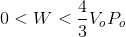All College Physics Resources
Example Questions
Example Question #1 : Thermodynamics
A 




If the specific heat of the steel is 

This is a simple pairing of two 
We will make the left side for the steel and the right side for water. Thus:
Solving for 

Example Question #1 : College Physics
A steel beam is 


We need to use the equation for thermal expansion in order to solve this problem:
We are given:


We need to calculate 
Now we have enough information to solve for the change in the length of steel.
Example Question #1 : College Physics
What is the change in entropy for 


Latent heat of fusion for water:
Specific heat of water:
Specific heat of ice: 
- We must account for two separate processes since the ice is melting and then warming. The basic formula to calculate the change in entropy at a constant temperature when the ice melts is:
Where 




Now we plug in our known values into the equation:
Mass
Latent heat of fusion
Temperature
And we get the change in entropy for the ice melting at a constant temperature, which is
- Now we need to calculate the change in entropy for the liquid water warming from
to
.
The basic formula to calculate the change in entropy at a changing temperature when the water warms is:
Next we substitute in an equation for 

next we integrate this equation to get:
Next we plug in the values we know and solve for the change in entropy:
Final temperature of water
Initial temperature of water
Specific heat of water
Mass of water
Now we get the change in entropy for the water warming to be
We add our two entropy values together to find the total change in entropy to be:
Example Question #1 : Thermodynamics
If an ideal gas has its temperature doubled while its volume is cut in half, what happens to its pressure?
The pressure decreases by a factor of
The pressure increases by a factor of
There is no change in the pressure
The pressure increases by a factor of
The pressure increases by a factor of
In this question, we're told that an ideal gas undergoes a change in its temperature and volume, and we're asked to determine how its pressure changes.
First, we'll need to recall the ideal gas equation.
Then, we can rearrange to isolate the term for pressure.
From this expression, we can see that if we double the volume, the pressure will double. Also, cutting the volume in half would double the pressure as well. Therefore, doing both of these would cause the pressure to increase by a factor of 
Example Question #2 : Thermodynamics
What volume does 
The first step in this question is to convert the mass of oxygen given into moles.

Next, it's important to recognize that we'll need to use the ideal gas equation. Since we're told that the gas is under STP (standard temperature and pressure), we have everything we need to solve for volume.
Example Question #1 : Thermodynamics
What is true regarding the work performed by a system whose volume remains constant and undergoes an increase in pressure?
The work done by the system increases the temperature of the system
The system does work on its surroundings
The system does no work
The surroundings does work on the system
The system does no work
To answer this question, it's important to remember how work is defined when dealing with volume and pressure.
The equation for the work done by a gas on its surrounding is as follows.
With this equation in mind, we can see that the only time work is performed is when there is a change in volume. Since we're told in the question stem that the volume remains constant as the pressure is increased, the final volume will be equal to the initial volume. Thus, the 

Example Question #2 : Thermodynamics
Suppose that a piston contains a gas which begins at a certain pressure and volume. First, the gas in the piston undergoes an isothermal expansion to triple its initial volume. Next, the gas undergoes an isobaric contraction back to its original volume. Finally, the gas undergoes an isochoric increase in pressure until it has returned to its original pressure. Which of the following is a true statement regarding the work done by this gas?
In this question, we're told the process that a gas takes through various changes in conditions. From this information, we're asked to find a true statement regarding the work done by the gas in this process.
At its initial point, we can define the gas as having an initial pressure and volume as 
First, we're told that the gas goes through an isothermal expansion to triple its volume. Recall that an isothermal process is one in which the temperature of the gas doesn't change. Moreover, because we're dealing with an ideal gas, we can see from the ideal gas equation that a constant temperature means that a changing volume must by accompanied by a change in pressure. Since the volume is being increased by a factor of three, we know that the pressure must be decreasing by a factor of three. Thus, right after the isothermal expansion has finished, the gas will have a pressure and volume of 
Next, we're told that the gas undergoes an isobaric contraction back to its original volume. Thus, we know from this that the pressure won't change, but the volume will return to its original value. Consequently, once the isobaric contraction has come to completion, the gas will have a pressure and volume of 
Finally, we're told that the last process is an isochoric increase of the pressure back to its original amount. Isochoric means that the volume doesn't change in this process. Hence, the gas will now be returned to its original pressure and volume of 
If we were to show this on a pressure vs. volume curve, it would look like the following.

Remember that any pressure-volume work done by a gas is the area under the curve of a pressure vs. volume diagram. For a cyclic process, as the one in this problem, the work done by the gas will be the area within the above image.
For this problem, we don't need to calculate the exact amount of work done. Instead, we can get a rough estimate by first realizing that the structure shown in the above diagram is close to forming a right triangle, but it is slightly smaller. Thus, if we can use the left and bottom sides of the image above to calculate the area that would be enclosed by a right triangle, we can infer that the work done in this problem will be slightly less than the calculated area.
The area for a triangle is the following.
So, for the image shown above, the bottom length is 

Finally, since this represents the area for a right triangle, we know that the actual work done is less than this. Hence, the correct answer is:
Example Question #1 : Isothermal Processes
An ideal gas with an initial pressure of 


We know that with the ideal gas law that:
We also know with isothermal processes, that the work done on a gas is:
Since the number of moles and the temperature is not given to us in the question, we can make a substitution with the ideal gas law to get:
Now we can plug in our known values into the equation to solve for the amount of work energy transferred through the isothermal process.
Example Question #1 : College Physics
A laser outputs light of a single color with photon energy 
Halving the wavelength is equivalent to doubling the frequency since for a light wave the wavelength and frequency are related by 



Example Question #2 : College Physics
A monochromatic light source is incident on a neutral density filter which decreases the light source's intensity by a factor of 3 but does not change its color. What happens to the energy of a photon in the less intense beam?
The photon energy decreases by a factor of 3.
None of these
The photon energy increases by a factor of 3.
The photon energy decreases only slightly.
The photon energy decreases by a factor of 9.
None of these
The photon energy does not change since the color of light (and therefore the frequency of the light) does not change.
All College Physics Resources