All MCAT Biology Resources
Example Questions
Example Question #2 : Substitution And Elimination Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
III, only
II and III
I and II
I and III
I, only
I and III
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
Example Question #11 : Reaction Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In the rate limiting step of reaction 2, which of the following describe the intermediate chemical species?
I. It has sp2 hybridization
II. It is trigonal planar
III. It exhibits bond rigidity, limiting rotation
I and III
II and III
III, only
I, II, and III
I and II
I and II
Reaction 2 is an E1 reaction, in which the rate limiting step is the formation of the carbocation intermediate. The carbocation intermediate has three single bonds and a positive charge on the central carbon; thus, it has sp2 hybridization, a planar structure, and free rotation about its single bonds. Bond rigidity is only observed with the presence of pi bonds.
Example Question #41 : Reactions
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Reaction 1 proceeds more quickly, owing to a more stable carbocation
Reaction 1 proceeds more slowly, owing to a less stable carbocation
Reaction 1 only proceeds with a stronger nucleophile
Reaction 1 proceeds more quickly, owing to a decrease in steric hindrance
Reaction 1 proceeds more slowly, owing to a higher activation energy
Reaction 1 proceeds more slowly, owing to a higher activation energy
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Example Question #13 : Reaction Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
IV, only
I and II
II, only
III, only
I, only
IV, only
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Example Question #11 : Substitution And Elimination Mechanisms

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?


None of these



The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

Example Question #3 : Other Groups
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Reaction rate in this reaction is not determined by concentration
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Example Question #1 : Mechanisms And Intermediates
When exposed to a good nucleophile, which molecule will most readily undergo an 

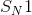

Example Question #61 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following compounds can be oxidized to form a ketone?
2-methyl-2-butanol
Methanol
Propyl alcohol
3-pentanol
3-pentanol
An alcohol can be oxidized and form a ketone only if the alcohol is a secondary alcohol. Tertiary alcohols cannot be oxidized and primary alcohols are oxidized to form aldehydes and carboxylic acids.
3-pentanol is a secondary alcohol, while 2-methyl-2-butanol is a tertiary alcohol. Methanol and propyl alcohol are both primary alcohols.
Example Question #61 : Organic Chemistry, Biochemistry, And Metabolism
What reagent would be best suited to accomplish the transformation shown below?

Methyl magnesium bromide,
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

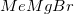
Example Question #2 : Organic Chemistry
Imagine the H-NMR spectroscopy of a propane molecule.
How many peaks represent the 2-carbon?
3
6
2
7
7
In order to determine how many peaks will be associated with the hydrogens of this carbon, you need to determine how many neighboring hydrogens surround the central carbon. Both of the terminal carbons have three hydrogens, so there are six total hydrogens neighboring the central carbon.
Since the number of peaks is given by the number of neighboring hydrogens plus one, there will be seven peaks on the spectrum for the 2-carbon. This is known as a septet.
Certified Tutor
Certified Tutor
All MCAT Biology Resources













