All MCAT Biology Resources
Example Questions
Example Question #1561 : Mcat Biological Sciences
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following proteins would be least affected by the introduction of mercaptoethanol?
A protein with no quaternary structure
A protein that has no cysteine amino acid residues
A protein that can not form alpha-helices
A protein with no proline amino acid residues
A protein that has no cysteine amino acid residues
Disulfide bonds are disrupted by the introduction of mercaptoethanol. Disulfide bonds are created by the interaction of two cysteine amino acids on different parts of the amino acid chain during the development of tertiary protein folding. As a result, a protein with few to no cysteine amino acids would be least affected by mercaptoethanol.
Alpha-helices are linked to secondary structure, and do not involve disulfide bonds. Similarly, quaternary structure is not determined by disulfide bonds; a protein without quaternary structure could still have disulfide bonds, which would be disrupted by mercaptoethanol. Proline is not involved in disulfide bonds, and its frequency would not affect the potency of mercaptoethanol to the protein.
Example Question #11 : Macromolecules
Which of the following forms of valine would be expected to exist under extremely acidic conditions?





At low pH levels, we expect amino acids to exist in their cationic form. At a pH level equal to the isoelectric point (pI) we expect amino acids to exist as zwitterions, and at high pH levels we expect them to exist in their anionic forms.
Low pH causes protonation of the amino groups; high pH causes deprotonation of the carboxyl groups.
Example Question #11 : Proteins
Polypeptides are molecules that contain multiple __________.
lipids
nucleic acids
amino acids
phosphates
monosaccharides
amino acids
Polypeptides are made from individual amino acids through formation of peptide bonds.
Monosaccharides are the fundamental units for carbohydrates, while fatty acids come together to form lipids. Nucleotides and phosphates are key components of the nucleic acids, RNA and DNA.
Example Question #11 : Amino Acids
What type of amino acid will have an isoelectric point above 7?
An amino acid with a polar side chain
An amino acid that is positively charged at a pH of 5
An amino acid with a basic side chain
An amino acid with no carboxylic acid
An amino acid with a basic side chain
The isoelectric point is the pH where the amino acid solution is electrically neutral. In acidic conditions, the carboxylic acid and amino terminus will both be protonated. This results in a positive charge (due to the amine being protonated). In basic conditions, both ends are deprotonated, resulting in a negative charge.
If an amino acid is basic, that means that the pH must be above 7 in order to deprotonate the amine in the side chain. Only then will the amino acid be electrically neutral. All basic amino acids (three of them) have an isoelectric point above a pH of 7. All other amino acids have an isoelectric point at a pH below 7.
Example Question #12 : Amino Acids
A polar amino acid in a highly basic solution is titrated with a strong acid. When will exactly half of the amino acid molecules be negatively charged?
At the second equivalence point
At the first half equivalence point
At the isoelectric point
At the second half equivalence point
At the first half equivalence point
The amino acid is polar, so we do not need to worry about charges in the side chain. Since the amino acid is starting in a highly basic solution, we know that the amino acid is deprotonated at both termini. That is, the amino terminus is neutral and the carboxyl terminus is negative. This results in a net charge of -1 (at the carboxyl end). The first half equivalence point upon titration will be seen when half of the amino acids are neutral and half of the amino acids are negatively charged. The full first equivalence point will show all molecules with a neutral charge, while the second full equivalence point will show all molecules with a positive charge due to protonation of the amine.
Example Question #13 : Amino Acids
A polar amino acid in a highly basic solution is titrated with a strong acid. Which pH is the best prediction for the isoelectric point of this amino acid?
1.0
5.4
7.0
8.2
5.4
Only basic amino acids have an isoelectric point above a pH of 7. All others will have an isoelectric point below a pH of 7.
Since the amino acid is polar, it will have an isoelectric point below a pH of 7. Since a pH of 1 is extremely acidic, we would expect both ends of the amino acid to be protonated at that pH. As a result, a pH of 5.4 is more appropriate when predicting the amino acid's isoelectric point.
Example Question #14 : Amino Acids
Which of the following amino acids contain(s) a hydrophilic functional group in its side chain?
I. Serine
II. Valine
III. Phenylalanine
IV. Tyrosine
V. Threonine
I, II, III, IV, and V
I and IV
I, IV, and V
II, III, and IV
I, IV, and V
Serine and threonine are classified as hydrophilic amino acids and contain hydroxyl (-OH) groups in their side chains. Tyrosine, although it is considered hydrophobic, does contain a hydrophilic hydroxyl group in its side chain. The answer is I, IV, and V, as all of these contain hydrophilic functional groups.
Example Question #1571 : Mcat Biological Sciences
All amino acids have at least two pKa values, one corresponding to the carboxylic acid, and one corresponding to the amine functionality. Some amino acids with polar side chains also have a pKa associated with the sidechain functionality.
Phenylalanine has pKa values of 2.58 (carboxylic acid) and 9.24 (NH2).
Arginine has pKa values of 2.01 (carboxylic acid), 9.04 (NH2), and 12.48 (side chain).
Valine has pKa values of 2.29 (carboxylic acid) and 9.72 (NH2).
For this problem, consider a molecule made of up of three amino acids, as described below.
HO-phenylalanine-arginine-valine-NH2
What would the overall charge of this molecule be at a pH of 7?
Phenylalanine:
Since the phenylalanine residue is at the C-terminus end of the molecule, only its carboxylic acid pKa is relevant, as its amine is involved in a peptide bond with arginine. At a pH of 7 (well above the carboxylic acid pKa of 2.58), the C-terminus carboxylic acid would be deprotonated and have a charge of 
Arginine:
For the middle amino acid, arginine, the only relevant pKa is that of its side chain since both its carboxylic acid and amino groups are involved in peptide bonds with neighboring amino acids. Since the side chain of arginine would be protonated at a pH of 7 (well below the sidechain pKa of 12.48), this amino acid would have a charge of 
Valine:
Finally, for valine, the relevant pKa to consider is the NH2 pKa of 9.72. At pH 7, this would also be protonated, resulting in a charge of 
The overall charge of the molecule at pH 7 would be 
Example Question #1572 : Mcat Biological Sciences
How many moles of base are required to fully deprotonate glutamic acid in its most acidic form (shown below)?

Zero
Two
Four
Three
Five
Three
One mole is base is required to deprotonate each carboxylic acid or amine, until the amino acid exists in its most deprotonated and basic form. First, two moles of base would be needed to deprotonate the two acid groups on glutamic acid (one depicted on each end). Next, a third mole would be needed to deprotonate the amine to its neutral state. Three total moles is the correct answer.
Example Question #17 : Macromolecules
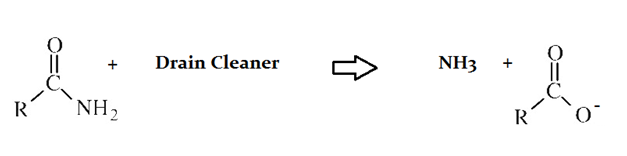
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In reaction 1, an organic acid forms as a product of the reaction of the original protein and drain cleaner. What quality of the resulting anion contributes most to the acidity of the product?
Basicity of the side chain
Resonance stabilization
Electronegativity of the carboxy terminus
Polarity of the C-O bond
The lone pair of electrons on the original nitrogen
Resonance stabilization
The resonance of the two C–O bonds that result after deprotonation of an organic acid is the major contributor to anion stability.
All MCAT Biology Resources