All MCAT Biology Resources
Example Questions
Example Question #202 : Organic Chemistry, Biochemistry, And Metabolism
An enzyme that cleaves disulfide bridges would most disrupt a protein containing which amino acid sequence?
Tyr–Cys–Cys–Thr–Val–Leu
Cys–Leu–Val–Tyr–Tyr–Thr
Val–Leu–Leu–Cys–Tyr–Thr
Tyr–Cys–Val–Val–Leu–Thr
All of the answers would be equally affected
Tyr–Cys–Cys–Thr–Val–Leu
Disulfide bridges are made between two cysteine amino acids. An enzyme that cleaves disulfide bonds would disrupt a protein containing the most cysteine residues; therefore, Tyr–Cys–Cys–Thr–Val–Leu is the correct answer.
Example Question #1 : Proteins
Which of the following is an example of the secondary structure of a protein?
Hydrogen bonding between an amine and carbonyl group
Hydrogen bonding between R groups
Disulfide bonds between cysteine residues
Peptide bonding between amino acids
Hydrophobic interactions
Hydrogen bonding between an amine and carbonyl group
By definition, the secondary structure of a protein is the hydrogen bonding between the amine and carbonyl groups in the amino acid chain. This usually occurs in the form of alpha-helices or beta-pleated sheets.
The linear sequence of the amino acids formed by peptide bonds is the primary protein structure. Interactions of R groups determines the tertiary structure. These interactions can be in the form of disulfide bonds, hydrogen bonding, or hydrophobic interactions.
Example Question #211 : Organic Chemistry, Biochemistry, And Metabolism
You are given the amino acid sequence Ala-Gly-His-Tyr. This is an example of which level of protein structure?
Quaternary
Secondary
None of the other answers are correct
Primary
Tertiary
Primary
Primary protein structure refers to the linear sequence of amino acids, which is the information given in this question. Secondary structure includes interactions between nearby parts of the linear chain, and includes the common examples of alpha-helices and beta-pleated sheets. Tertiary structure is the protein's three-dimensional shape, and quaternary structure refers to interactions between tertiary subunits.
Example Question #212 : Organic Chemistry, Biochemistry, And Metabolism
The term "denaturation," when used in conjunction with proteins or nucleic acids, refers to a change in structural characteristics primarily due to __________.
changes in primary structure
the binding of toxic compounds
the disruption of non-covalent bonds
the disruption of covalent bonds
the disruption of non-covalent bonds
The denaturation of proteins and nucleic acids occurs due to the disruption of non-covalent bonds, especially hydrogen bonds. In the case of nucleic acids, covalent bonds can be disrupted by specific enzymes, but this is not a form of denaturation. Changes in the primary structure of nucleic acids and proteins simply result in the net production of different proteins due to sequential changes of amino acids and nucleotides, not denaturation. Toxic compounds can interfere with nucleic acid and protein formation, but they do so by interrupting the non-covalent forces of each, such as hydrophobic forces and hydrogen bonding. Thus, the correct answer is the disruption of non-covalent bonds.
Example Question #44 : Proteins
Nuclear transport is a very important concept of study in modern cellular biology. Transport of proteins into the nucleus of an organism requires energy in the form of GTP, which is attached to a protein called Ras-related Nuclear protein (RAN).
RAN is a monomeric G protein found in both the cytosol as well as the nucleus and its phosphorylation state plays an important role in the movement of proteins into and out of the nucleus. Specifically, RAN-GTP and RAN-GDP binds to nuclear import and export receptors and carries them into or out of the nucleus. They also play a role in dropping off cargo that import and export receptors hold onto. RAN's functions are controlled by two other proteins: RAN guanine exchange factor (RAN-GEF) and RAN GTPase activating protein (GAP). RAN-GEF binds a GTP onto RAN, while RAN-GAP hydrolyzes GTP into GDP. As a result, there is a RAN-GTP and RAN-GDP concentration gradient that forms between the cytosol and nucleus.
One of the main roles of RAN is to bind to nuclear import and export receptors and carry them into or out of the nucleus. Given that import and export receptors are proteins, what can we say about the cooperativity displayed by RAN when it comes to binding to import and export proteins?
No cooperativity
Both negative and positive cooperativity
Positive cooperativity
Negative cooperativity
There is not enough information to determine what type of cooperativity exists
No cooperativity
Binding cooperativity occurs when binding of one substrate increases or decreases the affinity for the other substrates. For cooperativity to work, the protein in question must have multiple subunits, therefore being at least a dimer. RAN is a monomer, and therefore cannot show any cooperativity.
Example Question #1 : Enzymes And Enzyme Inhibition
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
Carbonic anhydrase is an organic enzyme. Which of the following is true of carbonic anhydrase? Assume no CO2 or bicarbonate is lost in the reaction.
I. It lowers the activation energy for the conversion of carbon dioxide to carbonic acid
II. It shifts the equilibrium toward carbon dioxide in experimental conditions
III. It modifies chemical species at its allosteric site
I and III
III, only
I and II
I, II, and III
I, only
I, only
Only choice I is correct. Carbonic anhydrase lowers the activation energy of a chemical reaction, as does any catalyst. Thermodynamics, including equilibria, are not modified by catalysts, so choice II is incorrect. Choice III is also incorrect, as an allosteric site is typically used to bind regulators of enzymes to induce conformational changes, while an active site would be where the actual catalysis takes place.
Example Question #2 : Enzymes And Enzyme Inhibition
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be optimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
The observed cooperativity of oxygen binding to hemoglobin can be explained by changes in shape to the hemoglobin molecule upon oxygen attachment. What kind of change would this be considered?
Primary
Allosteric
Competitive
Noncompetitive
Uncompetitive
Allosteric
Hemoglobin reacts with an allosteric change to oxygen binding, because the shape of the molecule changes. In fact, oxygen is considered a "homotropic" allosteric regulator because it is the normal substrate for hemoglobin, and affects its changes on that molecule by binding to its active site.
Example Question #2 : Enzymes And Enzyme Inhibition
Cryptosporidium is a genus of gastrointestinal parasite that infects the intestinal epithelium of mammals. Cryptosporidium is water-borne, and is an apicomplexan parasite. This phylum also includes Plasmodium, Babesia, and Toxoplasma.
Apicomplexans are unique due to their apicoplast, an apical organelle that helps penetrate mammalian epithelium. In the case of cryptosporidium, there is an interaction between the surface proteins of mammalian epithelial tissue and those of the apical portion of the cryptosporidium infective stage, or oocyst. A scientist is conducting an experiment to test the hypothesis that the oocyst secretes a peptide compound that neutralizes intestinal defense cells. These defense cells are resident in the intestinal epithelium, and defend the tissue by phagocytizing the oocysts.
She sets up the following experiment:
As the neutralizing compound was believed to be secreted by the oocyst, the scientist collected oocysts onto growth media. The oocysts were grown among intestinal epithelial cells, and then the media was collected. The media was then added to another plate where Toxoplasma gondii was growing with intestinal epithelial cells. A second plate of Toxoplasma gondii was grown with the same type of intestinal epithelium, but no oocyst-sourced media was added.
Where is the likely site of the neutralizing toxin synthesis in cryptosporidium cells?
Nucleus
Smooth endoplasmic reticulum
Nucleolus
Mitochondria
Ribosomes
Ribosomes
The passage specifies that the neutralizing agent is a peptide. Ribosomes synthesize peptides. Nuceloulus may have been a tempting answer, but is where ribosomes are synthesized, not peptides.
Example Question #1 : Enzymes And Enzyme Inhibition
Of the following statements, which is true regarding the change in free energy (ΔG) of a reaction?
When ΔG is zero, the system is at equilibrium.
ΔG predicts the rate of a reaction.
Two of these answers are correct.
ΔG is a measure of whether a reaction is spontaneous.
Two of these answers are correct.
Gibbs Free Energy (G) is a measure of the capacity of a system to do useful work as it proceeds to equilibrium. ΔG measures the spontaneity of a reaction; a negative value for ΔG indicates a spontaneous reaction, a positive value indicates a non-spontaneous reaction, and a value of zero indicates a reaction at equilibrium. ΔG does not predict enzyme kinetics; it only predicts thermodynamics, thus, two of the answers are correct.
Example Question #1 : Enzymes And Enzyme Inhibition
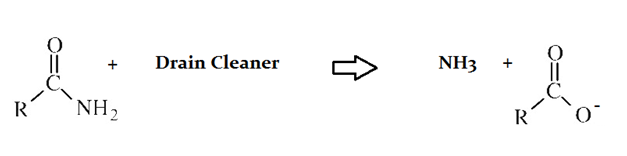
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Protein that forms the hair discussed in the preceeding passage is considered strucutral protein. Functional proteins, such as enzymes, are the other major class. Which of the following is true of enzymes?
They lower activation energy by changing the equilibrium position of a reaction.
They lower activation energy by providing an alternate reaction mechanism.
They lower activation energy by changing the products of a reaction.
They raise activaiton energy to prevent the reverse reaction from occuring as quickly.
They only lower activation energy of the original reaction mechanism.
They lower activation energy by providing an alternate reaction mechanism.
Enzymes are biological catalysts that function to lower activation energy via an alternative reaction pathway. They never alter the equilibrium of the reaction they impact.
All MCAT Biology Resources