All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #2 : Reaction Chemistry
When 15.5 grams of 

We must first calculate the molecular weight of 
For every mole of 

Therefore, 0.1092 moles of 
Example Question #3 : Reaction Chemistry
When 20.0 grams of 

We must first calculate the molecular weight of 
For every 1 mole of 

Therefore,the number of moles of 

Example Question #143 : General Chemistry
What is the smallest whole-number coefficient that can be designated to the water molecule that would balance the chemical equation given?
The number of atoms on the reactant side of the chemical equation are:
The number of atoms on the product side of the chemical equation are:
In a balanced chemical equation, the total number of atoms of each element must be equal on the reactant and product side of the chemical equation. There is one more oxygen and two more hydrogen atoms on the reactant side of the chemical equation. Giving the water molecule on the product side of the chemical equation would balance the chemical equation so that both sides contain six oxygens and five hydrogens:
Example Question #144 : General Chemistry
What is the smallest whole number coefficient that can be designated to the 
The process of balancing a chemical equation is called "balancing by inspection" which is done by trial and error. The easiest way to approach the problem given is by balancing the species containing an element with the most complicated formula occurring in one reactant and one product. In this case, we can either begin by balancing the carbon or hydrogen in the propane molecule. Let's start with the carbon by adding a coefficient of 3 to the carbon dioxide molecule on the product side of the equation which gives:
Let's now balance the hydrogens by adding a coefficient of 4 to the product side of the equation:
Lastly, we can balance the number of oxygens in the reaction:

Example Question #141 : General Chemistry
If 


Based on the molecular equation, for every 1 mole of 


We must calculate the molecular weight of 
To calculate the number of grams of 

Example Question #11 : Chemical Equations
If 


Based on the molecular equation, for every 4 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Example Question #141 : Gre Subject Test: Chemistry
If 


Based on the molecular equation, for every 2 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Example Question #141 : General Chemistry
If 


Based on the molecular equation, for every 3 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Example Question #141 : General Chemistry
Based on the chemical equation given, which of the following compounds underwent reduction?
The type of chemical reaction given is called an oxidation-reduction reaction. In this type of reaction, there is a transfer of electrons from one element to another. The species gaining an electron is said to be reduced and the species losing an electron is oxidized. Manganese ion goes from a 

Example Question #1 : Principles Of Oxidation Reduction Reactions
Methane combusts in the presence of oxygen according to the following reaction:
Which of the following statements is true concerning the reaction?
Oxygen has a charge of 

Oxygen is oxidized in the reaction
Carbon has an initial oxidation state of
Carbon is oxidized in the reaction
Carbon is oxidized in the reaction
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen 



Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of 

In the a product, carbon is attached to two oxygens, each with a charge of 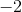

Since carbon went from an oxidation state of 
Certified Tutor
Certified Tutor
All GRE Subject Test: Chemistry Resources