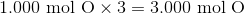All SAT II Chemistry Resources
Example Questions
Example Question #4 : Compounds
Which of the following are strong electrolytes?
All five
Four
Three
One
Two
Three
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Example Question #3 : Compounds And Molecules
How many of the following compounds are ionic?
All five
Three
Zero
One
Two
Two
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.


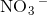



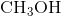




Example Question #11 : Sat Subject Test In Chemistry
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 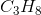
Example Question #2 : Bonding And Forces
In a solution, a weak electrolyte exists predominately as which of the following?
Ions
Molecules
Isotopes
Electrons
Polyatomic ions
Molecules
An electrolyte is a substance that when dissolved in a solution breaks up into ions. More specifically, an electrolyte breaks up into cations (positively charged ions) and anions (negatively charged ions). While strong electrolytes break up 100% into anions and cations, weak electrolytes break apart significantly less. Less than 10% of a weak electrolyte ionizes at all in solution. That means the 90%–99% of the weak electrolyte's molecules do not ionize. This is because the intermolecular forces that form between the solution and the weak electrolyte's molecules are not stronger than the intramolecular forces holding the molecule together. While solutions containing weak electrolytes contain both ions formed from the weak electrolyte ions and molecules of the weak electrolyte, they contain mostly molecules of the weak electrolyte.
Example Question #1 : Acid Base Reactions
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?



Example Question #2 : Acid Base Reactions
A scientist makes a solution by adding 0.2 grams of 






Example Question #1 : Oxidation Reduction Reactions
What is the oxidation number of nitrogen in 
First, note that the molecule does not have a charge, meaning that the oxidation numbers of each atom must add up to zero. Hydrogen has an oxidation number of 





Example Question #1 : Oxidation State
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: An 

BECAUSE
II: reduction is a loss of electrons
F, F
T, F
F, T
T, T (Statement II is not a correct explanation of Statement I)
T, T, CE
T, F
Statement II is false: reduction is a gain of electrons. Looking at statement I, the ion's charge would decrease by 3 in becoming a metal, which suggests that the ion gains 3 electrons. Thus, it is undergoing reduction.
Example Question #1 : Chemical Equilibrium
What is an expression for the equilibrium constant for the above equation?
The formula for the equilibrium constant is the product of the concentration of the products divided by the product of the concentration of the reactants. Since none of the reactants or products have coefficients, no exponents are needed in the expression. The phases do not affect the equilibrium constant in this example.
Example Question #1 : Empirical And Molecular Formulas
Vanillin is composed of 


What is the empirical formula of vanillin?
Refer to the following table for the atomic masses of the elements shown:
In order to calculate the empirical formula of vanillin from the given percent composition data, we can pretend we have a 100 g sample vanillin and from there estimate how many grams of each element make up the sample. For example, Vanillin is 

After finding the number of moles of each element in the hypothetical 100 g sample, we look at the ratio between the moles of each element by dividing all the samples by smallest number of moles. After finding the ratios of the samples, we are able to use the empirical formula of the vanillin by rounding up to the nearest whole number for each sample. If the ratios are not close enough to round up (like in this example), we multiply the ratios all by the same number until each number is equal to a whole number nicely. See calculations below:
We need whole numbers, so multiply each of the resulting numbers by 3 to get rid of the fractions.
This gives us 
All SAT II Chemistry Resources

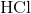




















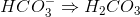




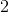








![\frac{[H_2CO_3]^3}{[H_2O]^2[CO_2]^2}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1073985/gif.latex)
![\frac{[H_2CO_3]}{[CO_2]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1073982/gif.latex)
![\frac{[CO_2][H_2O]}{[H_2CO_3]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1073983/gif.latex)
![\frac{[H_2CO_3]}{[CO_2][H_2O]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1073981/gif.latex)
![\frac{[H_2O]}{[H_2CO_3][CO_2]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1073984/gif.latex)