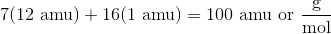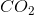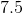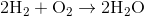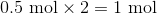All SAT II Chemistry Resources
Example Questions
Example Question #2 : Stoichiometry
Which pair of formulas represents the empirical formula and molecular formula for a certain compound?
To find an empirical formula, take a molecular formula and divide the subscript of each element by the greatest common factor of all the subscripts. In this case, the only pair that works is 
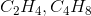

Example Question #1 : Molar Mass
What is the approximate percentage of carbon by mass in heptane, which has the molecular formula 
First, calculate the molar mass of heptane, which is
We can calculate the percentage of carbon by mass in heptane by creating a fraction of the mass of carbon over the mass of the entire molecule and converting it into a percentage:
For every 100 g of heptane, 84 g must come from carbon. This means that the percentage of carbon by mass is
Example Question #1 : Avogadro's Number
How many nitrate ions are in one mole of 
Note that there are two nitrate (
Example Question #2 : Avogadro's Number
How many hydrogen atoms are in one mole of 
In one molecule of 

Example Question #3 : Avogadro's Number
Calculate the number of aluminum ions in 

In order calculate the number of aluminum ions, we must first find the number of aluminum ions in the entire compound. In this case, there are two molecules of 


Example Question #4 : Avogadro's Number
How many atoms of chloride are in 0.2550 g of aluminum chloride, 
The formula for aluminum chloride is 





The total molecular weight is 130.33 g. Starting with the grams of 


Example Question #3 : Stoichiometry
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 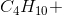



The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
Example Question #1 : Limiting Reagent
Suppose that 8 grams of of hydrogen gas and 16 grams of oxygen gas are ignited. What is the theoretical yield, in grams, of water? Assume hydrogen has an atomic mass of 1 and oxygen has an atomic mass of 16.
First, convert from grams to moles using the molar masses of the compounds. There are 4 mol of hydrogen gas and 0.5 mol of oxygen gas. Oxygen gas is clearly the limiting reagent (since there is more than twice as much hydrogen gas as oxygen gas), which means that the theoretical yield of water is
Water contains two hydrogen atoms and an oxygen atom, so it has a molar mass of
Hence, 1 mol of water would have a mass of 18 g.
Example Question #4 : Stoichiometry
Octane, 
If 114 g of octane are burned in the complete combustion reaction shown below, how many grams of water will be produced?
___



First, the equation should be balanced. This can be done with the coefficients 2, 25, 16, and 18.
Next, calculating the molar mass of octane shows that it has a molar mass of 114 g/mol. This means that 1 mole of octane is used in the reaction, and 9 times as many moles of water should be produced, meaning one of the products will be 9 mol of water. Water has a molar mass of 18 g/mol, so the total mass of water produced is 
Example Question #21 : Sat Subject Test In Chemistry
Suppose that 4 moles of 

Start by creating a skeleton equation for the decomposition reaction:
This can be balanced to give:
This means that 4 mol of reactant would produce 6 mol of oxygen gas. The question states that at STP, one mole of a gas takes up 22.4 L of space, so it is easy to find that 6 moles of oxygen gas would occupy a volume of 134.4 L:
Certified Tutor
Certified Tutor
All SAT II Chemistry Resources