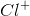All SAT II Chemistry Resources
Example Questions
Example Question #1 : Periodic Trends
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: Atoms in the alkaline earth metal group have smaller ionic radii than their atomic radii
BECAUSE
II: atoms in this group usually lose electrons in order to become ions, resulting in the ion lacking the outermost energy level present in the atom.
T, T, CE
F, F
T, F
T, T (II is not a correct explanation of I)
F, T
T, T, CE
The alkaline earth metals are the elements in group II, such as beryllium and magnesium. They want to have a complete octet in their outermost energy level, so they tend to lose two electrons to form the ionic state, which gives them the electron configuration of the noble gas in the previous energy level and thus a smaller ionic radius. Therefore, both statements are true and the second correctly explains the first.
Example Question #2 : Elements And Atoms
The elements with the smallest atomic radii tend to be found in which part of the periodic table?
The upper right corner
The upper left corner
The bottom right corner
The inner transition metals
The bottom left corner
The upper right corner
Atomic radius tends to increase down a group due to the addition of new energy levels; however, it decreases across a period due to increased shielding and increased nuclear mass. For these reasons, the elements with the smallest atomic radii would be in the upper right corner of the periodic table.
Example Question #1 : Sat Subject Test In Chemistry
An atom of phosphorus has 15 protons, 16 neutrons, and 15 electrons. What is the atom's mass number?
Protons and neutrons both have masses of approximately 1 amu, whereas the mass of an electron is negligible. Therefore, the mass number is equal to the number of protons and neutrons, which is 31.
Example Question #3 : Elements And Atoms
Which of the following has the electron configuration of ![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex)
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is 
Example Question #2 : Sat Subject Test In Chemistry
Which of the following variables identifies the sublevel that has three orbitals?
The 




Example Question #3 : Sat Subject Test In Chemistry
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?






Example Question #4 : Sat Subject Test In Chemistry
What is the molecular geometry of 
Octahedral
Tetrahedral
Square planar
Trigonal pyramidal
Square pyramidal
Square pyramidal
There are six electron domains in 


Example Question #5 : Sat Subject Test In Chemistry
Which of the following is the correct name of 
Dinitrogen monoxide
Dinitrogen pentoxide
Nitrogen dioxide
Nitrogen monoxide
Dinitrogen tetraoxide
Dinitrogen tetraoxide
In order to answer the question, it is important to first determine what kind of bonding is occurring in order to properly name the molecule. When nitrogen and oxygen form an 
Because a covalent bond is formed, we can determine that name of the molecule will be based on number of each atom in the molecule. There are two nitrogen atoms in the resulting molecule, so the prefix in front of the "nitrogen" part of the name will be "di-," meaning two. There are four oxygen atoms in the resulting molecule, so the prefix in front of the "oxygen" part of the name will be "tetra," meaning four.
The molecules names will be arranged alphabetically, and the second atom will have "-ide" as the suffix. Therefore, the correct answer is "dinitrogen tetroxide."
Example Question #1 : Complex And Polyatomic Ions
What is the formula for the compound formed from calcium and sulfate ions?
In order to answer this question correctly, it is important to have knowledge of the formation of ionic bonds, polyatomic ions, and the periodic table. First, it is important to notice that calcium ions and sulfate ions will form an ionic bond. Knowing this, it can be understood that valance electrons will be exchanged between the ions and therefore, oppositely charged ions that are attracted to each other will be formed.
Calcium is in the second column of the periodic table, so it will provide two ions to the sulfate polyatomic ion. This will form a calcium cation (

One calcium ion provides two electrons, which are both accepted by a sulfate polyatomic ion, so the ratio of calcium ions used to sulfate polyatomic ions used is 1:1. This means that only one of each ion is needed to form the compound, making the formula 
Initially it may be tempting to write the formula as 
Example Question #6 : Sat Subject Test In Chemistry
All of the following are properties of zinc. Which of the following are chemical properties of zinc?
A. Zinc produces hydrogen gas when placed in acid.
B. Zinc melts at 
C. The density of zinc is 
D. Zinc is a grey metal.
E. Zinc corrodes in moist air.
A, C, and E
B and C
C
A and E
B
A and E
Physical properties are aspects of matter that can be observed and measured without changing it. Chemical properties measure the potential for undergoing a chemical change. How zinc reacts to acid and in moist air are both chemical properties because the zinc is undergoing chemical change in each environment. The color, density, and melting point of the zinc can all be observed without changing the nature of the matter, so these are physical properties of zinc.
Certified Tutor
Certified Tutor
All SAT II Chemistry Resources