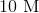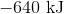All SAT II Chemistry Resources
Example Questions
Example Question #31 : Sat Subject Test In Chemistry
What is the mass percent of chromium in 
Use the values in the following table for the atomic masses of the elements shown.
In order to find the mass percent of a certain atom in a particular molecule, you must know the mass the atom contributes to the molecule and the total mass of the molecule. In this case we calculate the mass percent using the equation
Calculating the mass of 





Now we need to calculate the total atomic mass of the molecule. We can do this by identifying the atomic mass of each element in the molecule, multiplying it by the number of that type of atom in the molecule, and summing the results together.
We then plug in the values into equation to find the mass percent.
Example Question #1 : Gases
Suppose that an ideal gas at 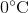

First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives

and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer, 
Example Question #32 : Sat Subject Test In Chemistry
How much faster is the rate of effusion of 

By Graham's Law, 


Example Question #34 : Sat Subject Test In Chemistry
30 mL of 1.0 M 
Recall that 

Example Question #1 : Thermochemistry And Kinetics
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 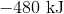
Example Question #34 : Sat Subject Test In Chemistry
A crucible is known to weigh 43.4273 g. Five students in the class determine the weight of the crucible by repeated weighings on a simple balance. Using the following information, which student has done the most accurate determination?
The student who has the accurate determination is that the student's whose average is closest to that of the "known" value of the crucible. Not be confused with precision, accuracy is how close the measured value is to the actual value, while precision is how close the measured values are to each other.
To answer this question, you need to calculate the averages of each students' weighings by summing them and dividing by five, since five measurements were taken.
Student 2's data averages out to 43.36, which rounds to 43.4. This average has the smallest difference from that of the "known" value for the crucible, 43.4273 g. Student 2's data is the correct answer.
Certified Tutor
Certified Tutor
All SAT II Chemistry Resources