All Organic Chemistry Resources
Example Questions
Example Question #1 : Help With Carboxylic Acid Reactions
Which of the following is derived from a carboxylic acid?
Acid anhydride
Imine
Aldehyde
None of these
Acid anhydride
A carboxylic acid can be synthesized directly into an acid anhydride by introducing the carboxylic acid to an acid chloride.
Example Question #2 : Help With Carboxylic Acid Reactions

What is the major product of the given reaction?





This question is asking us to determine what the product will be when a carboxylic acid is reacted with thionyl chloride, 


Example Question #11 : Carbonyl Reactants
What is the product of the reaction shown?


I
IV
II
III
III
The Grignard reagent also acts as a base, and will remove any protons that have a low-enough pKa. This means that the alpha hydrogen will be removed, and the resulting carboxylate ion is stabilized through resonance and is unlikely to react with the protonated Grignard reagent.
Example Question #1 : Help With Carboxylic Acid Reactions
What is the product of the reaction below?
This reaction is an acid-catalyzed esterification of a carboxylic acid. In the reaction the carbonyl is attacked by the alcohol oxygen, and the carbonyl is reformed by kicking off what was the hydroxyl group in the original carboxylic acid. What is left is an ester (of the form RCOOR', with R' containing the same number of carbons as was in the original alcohol).
Example Question #151 : Organic Chemistry, Biochemistry, And Metabolism
The compound below is reacted with 

The initially chiral carbon has an 




Example Question #581 : Organic Chemistry

What is the product of the reaction when the given molecule is introduced to an acidic solution?
None of these
2-bromo-4-hexene
2-bromo-4-hexanol
3-bromo-5-hexanol
4-bromo-2-hexene
4-bromo-2-hexene
In acidic solution, a hydrogen will be added to the hydroxy group of the given compound. This intermediate is a good leaving group. As it leaves, a hydrogen will be abstracted and a double bond will form. The bond is across carbons two and three becasue those two are more highly substituted than carbon 1, which is primary. The naming begins at the double bond, so the name is 4-bromo-2-hexene.
Example Question #19 : Carbonyl Reactants

Which reagent(s) would achieve the given synthetic transformation?
PCC
Chromic acid (formed in-situ via 



Example Question #53 : Reactions By Reactant
What is the product of the following reaction?
The key to answering this question is to realize that potassium permanganate 
Example Question #1 : Help With Alcohol Reactions
Which of the following compounds should be used to convert isopropyl alcohol to isopropyl bromide?


The following reaction is the bromination of a secondary alcohol:

Example Question #52 : Reactions By Reactant
When ethanol is reacted with 
Ethanal
Diethyl ether
1-Ethyne
1-Ethene
1-Propene
Diethyl ether
The correct answer is diethyl ether because this is the only ether compound listed.

Diethyl ether forms with the addition of a strong acid to ethanol.
Certified Tutor
All Organic Chemistry Resources

![\xrightarrow[]{SOCl_{2}}\: ?](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/479869/gif.latex)









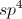




![CH_{3}(CH_{2})_{2}CH_{2}OH\xrightarrow[]{KMnO_{4}}\: ?](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/463035/gif.latex)












