All Organic Chemistry Resources
Example Questions
Example Question #241 : Organic Chemistry
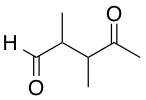
A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.
Ethane-1,2-diol + MeMgBr + H+ and heat
2 MeMgBr + H+
MeMgBr + H+
MeMgBr + H+ + ethane-1,2-diol
Ethane-1,2-diol + H+ and heat + MeMgBr
Ethane-1,2-diol + MeMgBr + H+ and heat
An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

Certified Tutor
All Organic Chemistry Resources




