All Organic Chemistry Resources
Example Questions
Example Question #1 : Organic Intermediates
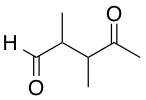
Identify the main functional groups in the pictured molecule.
Benzene, amide, aldehyde
Phenol, imine, ketone
Benzene, imine, aldehyde
Phenol, amine, ketone
Benzene, imine, aldehyde
1. Benzene
2. Imine
3. Aldehyde
Example Question #1 : Organic Intermediates
Which of the following transformations includes an enolate intermediate?
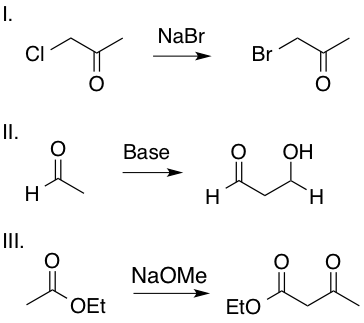
III only
I, II, and III
I and II
I and III
II and III
II and III
Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.

Example Question #2 : Organic Intermediates
Which of the following carbocation intermediates requires the least activation energy?


Cannot be determined



The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Example Question #1 : Identifying Carbocations
Rank the following carbocations from least to most stable.
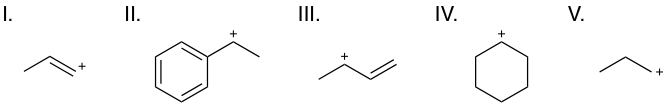
V < IV < III < II < I
V < IV < I < III < II
I < V < IV < II < III
I < V < IV < III < II
V < I < IV < II < III
I < V < IV < III < II
You many know that primary carbocations are less stable than secondary, which are in turn less stable than tertiary carbocations. This holds true in this question, and as such, V < IV. To evaluate compounds I, II, and III, however, we will need to determine their resonance capabilities.
We can compare the two special secondary carbocations, II and III—a benzyl carbocation and an allyl carbocation, respectively. The structure of these cations allow for the positive charge to be shared over several atoms via resonance as shown below:

We can see that the benzyl carbocation (II) is more stable than the allyl carbocation (III), as all its resonance forms have a secondary carbocation, and there are more resonance forms. As both share charge over multiple atoms, both are more stable than IV and V, which place charge on one atom only. Thus far, we can say: V < VI < III < II.
Lastly, we must consider I, a vinylic carbocation. This cation puts its negative charge on an sp2-hybridized carbon, and is the only example of this situation. Because an sp2 orbital has more overall s- character than an sp3 orbital, electrons are held closer to the nucleus. As the electrons feel more positive charge from the nucleus, the atom can accommodate more negative charge in general. We can think of this as a shift towards more electronegative behavior. As such, a positive charge will be more unstable on an sp2-hybridized carbon than on an sp3-hybridized carbon, and we can conclude than I is the least stable of all choices.
Thus, the correct answer is I < V < IV < III < II.
Example Question #241 : Organic Chemistry
Under which reaction mechanism can rearrangements occur?
Rearrangements generally require carbocations. Carbocations are only formed under mechanisms with unimolecular rate-limiting steps (i.e. carbocation formation). 
Example Question #241 : Organic Chemistry
A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.
Ethane-1,2-diol + MeMgBr + H+ and heat
2 MeMgBr + H+
MeMgBr + H+
MeMgBr + H+ + ethane-1,2-diol
Ethane-1,2-diol + H+ and heat + MeMgBr
Ethane-1,2-diol + MeMgBr + H+ and heat
An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

Certified Tutor
All Organic Chemistry Resources