All MCAT Physical Resources
Example Questions
Example Question #5 : Power
Two students (student X and student Y) lift a boulder vertically from point A to point B. Student X directly lifts the boulder from point A to point B, whereas student Y uses a pulley to lift the boulder. This allows student Y to apply a force (

The vertical distance between point A and point B is 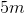
The power exerted by student Y is __________.
less than the power exerted by student X
equal to the force exerted by student Y times five
equal to the power exerted by student X
equal to force exerted by student Y times two
less than the power exerted by student X
To answer this question you need to know the definitions of work and power:
The units of work and power are Joules and watts, respectively. The passage states that the amount of time it takes to complete the task is same for both students; therefore, you can ignore the effects of time on power.
The passage also states that student Y applies less force. Since student Y applies less force, he has to expend less energy (work) than student X. This means that student Y also has to exert less power. A reduction in force will reduce work, and subsequently reduce power. We can thus conclude that student Y exerts less power than student X.
Example Question #6 : Power
Two students (student X and student Y) lift a boulder vertically from point A to point B. Student X directly lifts the boulder from point A to point B, whereas student Y uses a pulley to lift the boulder. This allows student Y to apply a force (

The vertical distance between point A and point B is 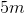
If student Y exerts a force of 

The first step is to find the force exerted by student X. The passage states that student Y exerts three times less force than student X; therefore, student X exerts three times more than student Y:
The second step is to find the amount of work done by student X.
Remember that student X directly lifts the boulder from point A to point B. This means that the distance the boulder travels is the vertical distance between point A and B (
The question states that time for the entire process is 
The correct answer is 
Example Question #1 : Optics
The focal point for a mirror is 56cm behind the mirror. Is the mirror concave or convex, and what is its radius of curvature?
Convex with radius of curvature of 112cm
Concave with radius of curvature of 112cm
Concave with radius of curvature of 28cm
Convex with radius of curvature of 28cm
Convex with radius of curvature of 112cm
Since the focal point falls behind the mirror it must be convex. The radius of curvature can be found using the focal length equation.
Example Question #1 : Mirrors And Lenses
A concave mirror has a radius of curvature of 0.85m. Where is the mirror's focal point?
The focal point cannot be found from the given information
Since the mirror is concave, the focal point will be in front of the mirror. The focal length is equal to one half of the radius of curvature.
Rc is the radius of curvature. Plugging in 0.85m for Rc allows us to solve for the focal length.
0.43m is equal to 43cm.
Example Question #1 : Mirrors And Lenses
A lens has a focal length of 





Since the focal length is negative, the lens is diverging.
The diopter of a lens is found through the following formula:
Since the focal length of the lens is 
Example Question #91 : Mcat Physical Sciences
Carbon dioxide and water are the two products formed from the combustion of a hydrocarbon. What type of intermolecular forces are present for water?
Hydrogen bonding and dipole-dipole attraction
London dispersion forces
Dipole-dipole attraction
Hydrogen bonding, London dispersion forces, and dipole-dipole attraction
Hydrogen bonding
Hydrogen bonding, London dispersion forces, and dipole-dipole attraction
Water exhibits hydrogen bonding. Each hydrogen atom is bond to a highly electronegative oxygen atom, resulting in a slight positive dipole on the hydrogen and a slight negative dipole on the oxygen. Within a solution of water, these dipoles can align, causing attractive forces between the hydrogens of one water molecule and the oxygen of another. Hydrogen bonding is a specialized form of dipole moment; since water has a permanent dipole moment, dipole-dipole attractions are present. Finally, like all molecules, water exhibits London dispersion intermolecular forces as well.
Example Question #92 : Mcat Physical Sciences
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


Of the following types of forces, which is most likely responsible for explaining the high vapor pressure of a very volatile chemical species?
Dipole-dipole bonds
Van der Waals forces
Coordinate covalent bonds
Hydrogen bonds
Polar covalent bonds
Van der Waals forces
Phase phenomena, such as vapor pressure, are best explained as a product of intermolecular forces. Strong intermolecular forces, like hydrogen bonds or dipole interactions, would make us expect weak vapor pressure, increasing attraction between molecules in the same phase and preventing transition. Large vapor pressures are more likely due to weak van der Waals intermolecular interactions, rather than stronger forces.
Example Question #92 : Mcat Physical Sciences
Which is not true of London dispersion forces?
They result from an asymmetrical distribution of electrons
They occur more frequently between larger atoms
They only exist between polar molecules
They are the weakest intermolecular force
They are caused by temporary dipoles
They only exist between polar molecules
London dispersion forces are weak, temporary attracting forces caused when electrons in adjacent atoms move into asymmetrical arrangements about their nuclei, forming temporary dipoles. Since electrons are constantly moving in any atom, both polar and nonpolar substances can develop temporary dipoles and experience London dispersion forces. These forces occur randomly as electrons form spontaneous polarized distributions, before immediately dissipating as the electrons move to new positions.
Example Question #93 : Mcat Physical Sciences
A student mislabels three jars containing three different molecules. The student frantically tries to find the identity of the molecules in each jar. He knows that the three possible molecules are methanol (





What is the identity of jar B?
Dichloromethane, because it only contains dipole-dipole interactions, the weakest intermolecular force
Propane, because it only contains London dispersion forces, the weakest intermolecular force
Dicholormethane, because it only contains dipole-dipole interactions, the strongest intermolecular force
Propane, because it only contains London dispersion forces, the strongest intermolecular force
Propane, because it only contains London dispersion forces, the weakest intermolecular force
The passage states that jar B has a boiling point of 
Many nonpolar molecules, such as propane, only contain London dispersion forces and lack all forms of dipole interaction. This results in very weak intermolecular interactions for these compounds, and will affect physical properties like vapor pressure, boiling point, and surface tension. Weak intermolecular forces will increase vapor pressure, decrease boiling point, and decrease surface tension.
Remember that polar molecules, such as methanol and dichloromethane, also contain London dispersion forces. They just contain much stronger forces (hydrogen bonding and dipole-dipole interactions) that make the London dispersion forces negligible.
Example Question #93 : Mcat Physical Sciences
Halogens start out as gases (fluorine and chlorine), but transition to liquid (bromine) and then to solid (iodine) when moving down group 17 of the periodic table. This is because __________.
the atoms lose their valence electrons more easily
the larger molecules have larger electronegativities, making them cluster closer together with one another
London dispersion forces increase as the molecules become larger
the atoms become heavier
London dispersion forces increase as the molecules become larger
The effects of London dispersion forces are best demonstrated by viewing the halogen group. The lighter halogens are gaseous at room temperature, but transition to liquid and then to solid when moving down the group (bromine is a liquid, and iodine is a solid). When moving down the group, the halogens become much larger. Larger atoms experience greater London dispersion forces due to increased surface contact and greater polarizability. These increased London dispersion forces are enough to raise the boiling points of the larger atoms, causing them to exist as liquids of gases at room temperature.
While it is true that the larger atoms are heavier and will lose their valence electrons more readily, these factors do not impact the phase of the compound.
All MCAT Physical Resources



























