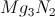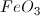All AP Chemistry Resources
Example Questions
Example Question #7 : Elemental Composition Of Pure Substances
Suppose the mass of an unidentified substance is determined to be 53.3% carbon, 11.1% hydrogen, and 35.6% oxygen. It is also discovered that the number of moles of this substance in a 100 gram sample is 1.1 moles. What is the empirical formula for this substance? What is the molecular formula?
Empirical formula:
Molecular formula:
Empirical formula:
Molecular formula:
Empirical formula:
Molecular formula:
Empirical formula:
Molecular formula:
Empirical formula:
Molecular formula:
Empirical formula:
Molecular formula:
To find the empirical formula, we have to determine a mole ratio of the elements in the substance, which we can get by dividing the mass percents of the elements by their respective atomic masses. Approximating:
To get an empirical formula we want to have whole number ratios, so we have to find a common whole number multiple or dividend that will make all of these pieces whole. A common strategy is to divide everything by the smallest number (in this case, 2.22).
We can now write our empirical formula using the above ratio for our subscripts:
To find the molecular formula, we need to use the molar mass. We are given that 100 grams of this substance is equal to 1.1 moles; dividing grams by moles gives a molar mass of 91 grams per mole.
The molar mass of is 45 grams per mole.
We divide the molar mass of the actual substance by the molar mass of the empirical formula:
Hence, 2 is the constant multiplier we apply to every subscript in our empirical formula, obtaining the final molecular formula:
Example Question #751 : Ap Chemistry
Suppose it is observed that 360 grams of carbon, 40 grams of hydrogen, 280 grams of nitrogen, and 320 grams of oxygen all react to form 1 kg of a new subtance. What is the empirical formula of that substance?
We are given a mass ratio of the elemental components, so all we have to do is divide each given mass by the respective atomic mass of its element. Approximately, the atomic masses of the elements in question are:
We now have to reduce this proportion to its smallest possible terms. Since they are all visibly multiples of 10, it makes sense to divide them by 10, yielding:
Since there are 3 carbons, and 3 is a prime number, this is the smallest whole number proportion we can have. Plug the numbers into a formula as subscripts, yielding the empirical formula:
Example Question #71 : Atomic Structure And Properties
What is the empirical formula of glucose?
The molecular formula for glucose is . The empirical formula is the most reduced formula. So think of the biggest number that: 6, 12, and 6 can be divided by. All of those numbers can be divided by 6, so we end up with 1, 2, 1 as our coefficients. That means we should have 1 carbon, 2 hydrogens, and 1 oxygen in the most reduced formula. Note that the 1:2:1 ratio of these elements is characteristic of all carbohydrates. They are hydrates (water) of carbon. Thus they contain one water molecule

Example Question #72 : Atomic Structure And Properties
Suppose that a compound contains 


In this question, we're given the elements that make up a given compound, and we're told the relative percentages of each element. We're asked to determine the empirical formula.
Though there are a few different ways to approach this problem, perhaps the easiest one is to first imagine that we're starting off with 
Now that we have the number of moles of each element, we can list our compound as follows.
Note that this is not yet the empirical formula of our compound, because an empirical formula has the simplest integer number for each of its elements. To arrive at our empirical formula, we can divide each element's number by the lowest number among them. In this case, sulfur's number is the lowest.
The numbers above are very close to whole number integers, so we can safely round them off. Thus, the empirical formula that we arrive at for this compound is the following.
Example Question #73 : Atomic Structure And Properties
A compound is composed of 13% carbon, 4.3% hydrogen, 30.4% nitrogen, and 52.2% oxygen. The mystery compound has a molar mass of 184 grams per mole. What is the molecular formula of the compound?
When finding molecular formulas, imagine a 100-gram sample of the compound. We can then use the percentages of each atom and convert them to grams.
The next step is to divide the given mass of each atom by its atomic mass. This will give you four separate molar values that you can compare to one another.
Next, you must divide each molar value by the smallest of the values. This will result in the molar ratios of each compound. In this case, carbon has the lowest molar value at 1.08. After dividing all four numbers by this value, we determine a molar ratio of 1:4:2:3 for carbon, hydrogen, nitrogen, and oxygen, respectively.
At this point, we have determined the empirical formula for the compound, however, we need to find the formula that gives a molar mass of 184 grams per mole. Start by calculating the molar mass of the empirical formula.
Since the empirical formula has a molar mass of 92 grams per mole, we need to multiply the empirical formula by 2.
This results in a molecular formula of .
Example Question #751 : Ap Chemistry
A neutral ionic compound consisting of a calcium ionic species and a hydroxide ionic species can be predicted to have which of the following molecular formulas?
Ca2OH
Ca2(OH)3
Ca(OH)3
Ca(OH)2
CaOH
Ca(OH)2
Here we are looking for the molecular formula of an ionic compound consisting of Ca and OH. We know that the molecule is neutral, so it must have a charge of 0. This question is really addressing one's knowledge of typical ionic species. Calcium ions are found only as Ca2+ and hydroxide ions (OH) are found only as OH–. Thus for these two species to exist in an neutral ionic compound the only possible formula is Ca(OH)2.
Example Question #752 : Ap Chemistry
What is the correct formula for a compound made of magnesium and nitrogen?
To find the correct formula, the charge contributions from each compound must cancel out to zero. Each magnesium ion has a charge of 



Example Question #753 : Ap Chemistry
What is the proper empirical formula for a compound composed of lithium and oxygen?
To find the correct formula, the charge contributions from each compound must cancel out to zero. Each lithium ion has a charge of 



Example Question #78 : Atomic Structure And Properties
Which of the following compounds has a percent composition of carbon, (approximately 63%)?
Butanol
C3H8
Glucose
Acetone
Acetone
Acetone, which as a formula of C3H6O has a total molecular weight of: 3(12) + 6(1) + 1(16) = 58g/mole, and the percent of this that carbon makes up is (3(12)/58) X100 = 63%.
Example Question #73 : Atomic Structure And Properties
What is the formula for the ionic compound iron(III) oxide?
When finding the formula for a neutral compound, you must make sure that the positive charges on the cation are cancelled out by the negative charges on the anion. Iron is a transitional element, and can create a couple of different cations depending on how many electrons it loses. The roman numeral III tells you the charge on the iron cation is 3+ (

This solution tells us the ratio of oxygen to iron: for every three oxygen, there are two iron.

Certified Tutor
All AP Chemistry Resources