All Physical Chemistry Resources
Example Questions
Example Question #1 : Properties Of Solids
Which of the following is true regarding solids?
None of these
Each solid requires a unique amount of energy to separate the molecules
Solids have high entropy
More than one of these
Each solid requires a unique amount of energy to separate the molecules
There are three main phases: solids, liquids, and gases. Solids molecules are closely packed in an organized, lattice structure, liquid molecules are more spread apart and more disorganized, and gas molecules are spread even farther apart from each other and are extremely disorganized. Each type of solid is made up of unique type of atoms. For example, magnesium metal is made up of magnesium atoms whereas copper metal is made up of copper atoms. Since they are made up of unique atoms, different types of solids have different interactions between atoms. Some have strong interactions whereas others have very weak interactions; therefore, the energy required to break these interaction and separate the atoms/molecules (melt) is different for each solid.
Recall that entropy is the level of disorder in a system. As mentioned, solids are highly organized structures; therefore, they will have the lowest entropy.
Example Question #2 : Properties Of Solids
You freeze a sample of nitrogen. Compared to the reactant, the end product has __________ density and __________ mass.
the same . . . the same
a higher . . . a higher
a lower . . . the same
a higher . . . the same
a higher . . . the same
Freezing the is process of converting a liquid to a solid. This question is asking about the freezing process of liquid nitrogen to solid nitrogen; therefore, the end product of the reaction is solid nitrogen. Recall that solids are more tightly packed. This means that the volume taken up by the molecules in solid is lower than in liquid; therefore, solids generally have a lower volume. Mass, on the other hand, depends on the number of molecules present. Phase changes do not alter the amount of molecules present. For example, the end product in this question (solid nitrogen) will have the same amount of molecules as its liquid counterpart; therefore, the mass doesn’t change when the phase changes.
Density is defined as follows.
Since its volume decreases and the mass stays the same, a solid will have a lower density than liquid. Note that water is an exception to this general rule, as solid ice has a lower density (higher volume) than the same mass of liquid water. This is due to the arrangement of its hydrogen bonds throughout its crystalline structure.
Example Question #1 : Phases And Properties Of Matter
An unknown molecule (molecule A), in its solid phase, is found to have a density of 

Solid will not fit in the container
No liquid will overflow
The container will be about halfway full with liquid
Liquid will overflow
Liquid will overflow
The dimensions of the cubic container are 
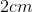

Recall that 


Example Question #1 : Solids And Liquids
Which of the following is true regarding the liquid phase?
I. Liquids have lower entropy than gases
II. Compared to solids, more energy is required to separate liquid molecules
III. Liquids have higher intermolecular forces than gases
I and II
I, II and III
III only
I only
I, II and III
Liquid is an intermediate phase, between solid and gas. Solids are characterized by tightly packed molecules in an organized manner whereas gases are characterized by greatly spread out molecules that are highly disordered; liquids lie somewhere in the middle. This means that they are more disordered than solids (more entropy) and less disordered than gases (less entropy).
The boiling point of a substance is always higher than the melting point; therefore, you always need higher energy to break the interactions between liquid molecules.
The key difference between a liquid and a gas is the distance between the molecules. Liquid molecules are closer together whereas gas molecules are spread apart. Intermolecular forces are the forces between adjacent molecules. Since they are highly spread apart, the gas molecules have lower intermolecular forces.
Example Question #1 : Phases And Properties Of Matter
Which of the following are responsible for the high boiling point of water, compared to molecules of similar size?
Positive charge
Molecular size
Dipole-dipole interactions
Hydrogen bonding
Two lone pairs of electrons
Hydrogen bonding
Hydrogen bonding occurs between water molecules. While intermolecular forces between molecules exist for most molecules, these forces are much weaker than the bond formed between a hydrogen from one molecule and an oxygen, fluorine, and/or nitrogen atom of another molecule. Since the molecules are held more tightly together, more energy is required to break those bonds. This results in a higher temperature required to boil water (boiling breaks bonds between molecules, and this causes the molecules to escape into the gas phase).
Example Question #3 : Phases And Properties Of Matter
An object is floating on water. Which of the following is true?
I. The object has a lower density than water
II. The buoyancy force would be higher if the object sank
III. The volume of fluid displaced would be higher if the object sank
I and III
II only
II and III
I and II
I and III
Recall than an object floats on water if it has a lower density than water. A floating or submerged object in a liquid has a gravitational pull (downward force) equal to the buoyancy force (upward force). The force due to gravity (gravitational pull) depends only on the mass of the object. In this case, the object can never be submerged because the density of the solid cannot be changed (unless the phase of the object is changed). To submerge, the object has to be placed in a liquid with LOWER density.
The volume of fluid displaced depends on the volume occupied by the object in the liquid. When an object floats, only part of the object is inside the liquid. This means that only part of the object’s volume is occupied inside the liquid (lower volume of fluid displaced). If the object were to be submerged (in a different liquid), the entire volume of the object would be inside the liquid and there would be an increase in the volume of fluid displaced.
Example Question #2 : Solids And Liquids
Fluid A and Fluid B flow through the same pipe. However, it is found that fluid A flows much slower than fluid B. What can you conclude about fluid A and fluid B?
Both of these
Fluid A has a higher coefficient of viscosity
None of these
The forces between layers in Fluid A is lower
Fluid A has a higher coefficient of viscosity
Viscosity is a measure of resistance to flow. A liquid with high viscosity is said to have strong forces between its layers, making it harder to flow whereas a liquid with low viscosity has weak forces between its layers, making it easier to flow. Viscosity is quantified by the coefficient of viscosity (higher the coefficient, higher the viscosity and higher the resistance to flow). The question states that fluid A has more trouble flowing; therefore, fluid A has higher coefficient of viscosity and stronger forces between layers.
Example Question #5 : Phases And Properties Of Matter
According to Poiseuille’s principle, the flow rate __________ as viscosity increases and the flow rate __________ as length of pipe increases.
increases . . . increases
increases . . . decreases
decreases . . . decreases
decreases . . . increases
decreases . . . decreases
Poiseuille’s principle quantifies the flow rate of a fluid through a pipe to numerous variables as follows.
where 




Example Question #6 : Phases And Properties Of Matter
One end of a pipe (End A) has twice the radius as the second end (End B). What can you conclude about the flow of fluid through this pipe?
Fluid will flow faster and have a higher pressure at End B
Fluid will flow faster and have a lower pressure at End A
Fluid will flow faster and have a higher pressure at End A
Fluid will flow faster and have a lower pressure at End B
Fluid will flow faster and have a lower pressure at End B
To solve this question, we need to use the continuity equation and Bernoulli’s equation. The continuity equation is as follows:
where 




Bernoulli’s equation is as follows
where 




Example Question #1 : Phases And Properties Of Matter
Why is liquid water more dense than ice?
Water crystalizes with greater average space between molecules
Water has metastable phases
The slope of the solid/liquid phase line for water is positive
None of these
Liquid water and solid ice have the same density
Water crystalizes with greater average space between molecules
While water exhibits hydrogen bonding and has metastable phases, these don't have anything to do with the density of water. The slope of the solid/liquid line is in fact negative. This tells something about the relative densities of the phases. For a phase diagram, moving along a line of constant temperature (vertically on a phase diagram tells us something about the densities. Moving up along the line indicates an increase in density (this makes sense, since gasses are the least dense, and occupy the bottom portion of a phase diagram). If you move along a constant temperature line, you will see that water has a higher density than ice, since the ordering goes vapor, ice, then water. This is a result of the packing in solid ice.
All Physical Chemistry Resources










