All Organic Chemistry Resources
Example Questions
Example Question #621 : Organic Chemistry
Consider the following reaction.


In this reaction, two molecules of an alpha-hydroxy acid are condensed with heat to form product. What functional group is created in this reaction?
Aldehyde
Ester
Ketone
Ether
Ester
For this question, we're shown the structures of both the reactants and products, and we're asked to identify which functional group is in the product.
First, let's go through the answer choices and define them. Then, we'll compare that definition with what we see in the product.
Aldehydes are functional groups in which a carbon atom is double bonded to an oxygen atom. The carbon atom must also be bound to at least one hydrogen atom, and to another hydrogen or carbon atom. Usually, aldehydes occur at the terminal ends of a molecule.
Ketones are similar to aldehydes. These functional groups contain a carbon atom double bound to an oxygen atom. In addition, the carbon atom is bound to two other carbon atoms. Thus, ketones tend to be found within a compound, as opposed to at the terminal ends.
Ethers are a class of functional group in which an oxygen atom is situated between two carbon atoms via a single bond.
Carboxylic acids are a functional group in which a carbon is double bound to one oxygen atom, and also single bonded to the oxygen of a hydroxyl group. This functional group does not occur in the product, but it does occur in the reactant.
Esters are a functional group in which a carbon atom is double bonded to an oxygen atom, and also single bonded to another oxygen atom. However, unlike carboxylic acids, esters do not contain a hydroxyl group. Instead, the oxygen atom is bound to another "R group," typically another carbon atom.
Now, let's go ahead and take a look at the product. Shown in red circles, we see that there are two ester groups in the product. Thus, the ester functional group is the correct answer.

Example Question #1 : Carbonyl Products
Which of the following reactions would NOT produce a carboxylic acid?
PCC is considered a weak oxidizing agent. The reaction of a primary alcohol with PCC would only yield an aldehyde, while reaction with a secondary alcohol will yield a ketone. PCC will not be used to generate carboxylic acids.
A stronger oxidation, like 

Example Question #1 : Carbonyls
Which one of the following compounds can produce a carboxylic acid only when reacted with sodium dichromate and sulfuric acid?
2-pentanol
2-methylpentan-2-ol
Pentanal
1-pentanol
2-pentanone
1-pentanol
After recognizing the reagents given, we know we need to begin with an alcohol. The alcohol choices differ only by how substituted they are.
For a carboxylic acid to form from a reaction with sodium dichromate and sulfuric acid, a primary alcohol needs to be available. Therefore, 1-pentanol is the correct answer.
Example Question #21 : Reactions By Product
When 3-chloroheptane undergoes malonic ester synthesis, the final product is __________.
None of the other answers
a ten-carbon ketone
3-ethylheptanoic acid
nonanoic acid
malonic ester
3-ethylheptanoic acid
The malonic ester is deprotonated at the most acidic hydrogen, the one on the carbon between the two oxygens. The electrons from that bond to the hydrogen form a carbon-carbon double bond while electrons from the oxygen-carbon double bond go to the oxygen atom. This is the enolate form. The chlorine leaves the 3-chloroheptane and the electrons from the carbon-carbon double bond bond to the carbon that the chlorine left. At the same time, the carbonyl is reformed. After deesterfication, 3-ethylheptanoic acid is formed.
Example Question #621 : Organic Chemistry
Which of the following is not a derivative of a carboxylic acid?
Aldehyde
All of these are carboxylic acid derivatives
Amide
Ester
Aldehyde
Aldehyde is not a derivative of carboxylic acid. Esters can be derived from carboxylic acids by reacting them with 



Example Question #22 : Reactions By Product
Which of the following reagents is needed to convert an amide into a carboxylic acid?
All of these
As a general rule, any carboxylic acid derivative can be converted into al carboxylic acid when reacting with
Example Question #622 : Organic Chemistry

What is the product of the given reaction?


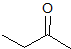



The hydrolyzation of a nitrile with hydronium leads to a carboxylic acid. Only one choice is a carboxylic acid.
Example Question #621 : Organic Chemistry
Please choose the best answer for the following question.
Which of the following reagents is best for converting a primary alcohol to a carboxylic acid?


PCC

Example Question #1 : Electrophilic Addition
What is the product of a hydroboration–oxidation reaction with 1-hexylcyclohexene?
3-hexylcyclohexanol
2-hexylcyclohexanol
Hexylcyclohexane
Cyclohexane
1-hexylcyclohexanol
2-hexylcyclohexanol
This reaction is an electrophilic addition reaction with an alkene. This is one of many alkene addition reactions that can add an -OH group onto your starting material. The key aspect of an hydroboration-oxidation reaction is the anti-Markovinikov addition to the double bond. The -OH group should be on the least substituted of the two carbons that originate from the double bond. In light of this information, the answer is 2-cyclohexanol.
Example Question #622 : Organic Chemistry

What is the product when the given starting compound is reacted with lithium aluminum hydride and acid?
2-butanol
1-butanone
No reaction will occur
2-butanone
1-butanol
2-butanol
This reaction involves a very strong reducing agent in lithium aluminum hydride, 
Certified Tutor
All Organic Chemistry Resources


















