All Organic Chemistry Resources
Example Questions
Example Question #1 : Help With Other Carbonyl Reactions
Which of the following statements is false?
A nucleophile attacks an epoxide to yield an alcohol
The catalyzed ring-opening of an epoxide in aqueous acid will yield a cis glycol
Treatment of an unsymmetrical epoxide with methanol and acid will result in nucleophilic attack at the more hindered carbon
The base-promoted ring-opening of an epoxide using aqueous NaOH will yield a trans glycol
Treatment of an unhindered epoxide with a Grignard reagent will result in a nucleophilic attack at the less hindered carbon
The catalyzed ring-opening of an epoxide in aqueous acid will yield a cis glycol
The statement "The catalyzed ring opening of an epoxide in aqueous acid will yield a cis glycol" is incorrect. These reaction conditions will yield a trans glycol. In fact, regardless of conditions, the opening of an epoxide will always yield a trans glycol (the two alcohol groups are on opposite sides).
Example Question #2 : Help With Other Carbonyl Reactions
All of the following electrophilic substrates can theoretically undergo substitution reactions, however, at different rates. Rank them from most to least reactive in the presence of a nucleophile.
![]()
I > IV > III > II
II > III > I > IV
III > I > II > IV
I > III > II > IV
II > IV > III > I
II > III > I > IV
As the first step in a substitution reaction involves a nucleophilic attack at an electrophilic carbonyl carbon, we must consider the varying reactivity of the electrophilic carbonyl center. Resonance diagrams, as well as an understanding of electronegativity, will help us understand the degree to which this effect is observed in a substrate.
Resonance diagrams for all four substrates show how electrons contained in the leaving group's heteroatom may be shared throughout the carbonyl system, effectively placing a partial negative charge on the electrophilic carbon. To determine which is the most electrophilic, we must identify the resonance diagram below that contributes the least to the overall molecule. This molecule will be least stable and most reactive.
Note: Remember, resonance diagrams show possible electron distributions, and a molecule exists as a weighted average of these possibilities, favoring the more stable ones.
Compound II is the most electrophilic substrate, as the lone pair on the central oxygen molecule must be shared between two carbonyls. The resonance forms below each contribute very little to the overall molecule. This is not the case in any other pictured substrate.
Now compare compounds I and III. Resonance for these molecules is essentially identical, with a nitrogen atom in compound I and an oxygen atom in compound III. We may conclude that the resonance form of compound III contributes less to the true existence of the molecule, as oxygen is more electronegative. The sharing of electrons will be less favorable in the resonance form of compound III than the resonance form of compound I.
For compound IV, both resonance structures are equally stable, and the molecule will exist as an average of both structures, placing a fair amount of electron density at the carbonyl carbon, drastically reducing the electrophilicity of the central carbon.

If this above explanation is confusing to you, you may also compare how good the leaving groups are. Acetate, the leaving group of compound II, is a stable ion and will readily leave in a substitution reaction. Methoxide is the next best leaving group, from compound III, followed by the negatively charged ethanamine leaving group from compound I. As 
The compounds, in order of reactivity, are II > III > I > IV.
Example Question #61 : Reactions By Reactant

Identify the missing intermediate in this acyl-substitution.
None of these
IV
II
I
III
III
After the initial protonation of the carbonyl-oxygen, nucleophilic attack by water occurs at the carbonyl-carbon, resulting in the positively-charged carbonyl oxygen accepting electrons from the carbonyl double bond, yielding an intermediate in which the only charge is found on the attacking water molecule (intermediate III). A proton is then quickly transferred to the chlorine, which dissociates as the carbonyl is reformed. Removal of the hydrogen bound to the carbonyl oxygen by another water molecule terminates the reaction. The reaction is favorable since carboxylic acids and carboxylates are less energetic than acid halides.
Example Question #4 : Help With Other Carbonyl Reactions
Predict the major product of the following reaction.

II
I
IV
III
I
Alkenes are saturated by hydrogen gas in the presence of a palladium catalyst. This type of reaction is termed catalytic hydrogenation and results in syn addition of hydrogen across a double bond. Molecules II, III, and IV have all undergone oxidation-reduction reactions and do not result from the given conditions. Molecule I is altered only by hydrogenation of the 2-3 double bond and is the correct answer.
Example Question #23 : Carbonyl Reactants
What is the final major product if this reaction proceeds in basic conditions?
1.
2.
3.
4.
As the reagents show, this is a two step reaction.
First, we use MCPBA, a common reagent used to make epoxide. Next, we introduce the methanol, which performs a nucleophilic attack on the epoxide. However, because the reaction is taking place in base, the methanol attacks the less substituted side of the epoxide. The methanol performs a nucleophilic backside attack, and so the stereochemistry becomes inverted. Work up is assumed throughout the reaction.
Example Question #62 : Reactions By Reactant
Which of the following organometallic reagents can produce 2-methylbut-3-en-2-ol from but-3-en-2-one?
I.
II.
III.
I, II, and III
III only
II and III
I only
I and II
I and II
This is a 



Example Question #61 : Reactions By Reactant
What is the product of the following reaction?






This question is asking us what the product will be when the reactant has been reacted with 

Example Question #1 : Organic Chemistry
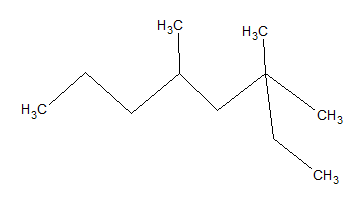
What is the IUPAC name of the given molecule?
3,3,5-trimethyloctane
None of these
2,2,4-trimethyloctane
3,3,5-trimethylnonane
4,6-dimethyl-6-ethylpentane
3,3,5-trimethyloctane
The longest carbon chain that can be formed is eight carbons. The base molecule is octane.
Using IUPAC rules, substituents should have the lowest possible numbers; thus, we start counting carbons from the right side rather than the left. If you count from the correct side, there are two methyl groups on carbon 3 and one on carbon 5. Thus, the name of the moleculue is 3,3,5-trimethyloctane.
Example Question #2 : Functional Group Reactions

How could you brominate the compound?
Hydrobromic acid
Bromine and peroxides
Bromine gas
Bromine and UV light
None of these
Bromine and UV light
The given molecule is an alkane. The only way to brominate an alkane is with bromine gas and UV light. The energy from the light serves to creat two radical bromines. These radicals are capable of bonding with alkanes. If the given compound were an alkene, either hydrobromic acid or bromine and peroxides would work.
Example Question #1 : Reactions By Product
Predict the absolute configuration about the double bond formed in the given E1 reaction.

E
No elimination reaction would proceed
Z
Racemic Z/E
E
Unlike E2 reactions, in which hydrogen abstraction occurs simultaneously with the dissociation of the leaving group (limiting the configuration of the reaction's product), E1 reactions occur in two distinct steps. The slow rate-determining step that must first occur is the dissociation of the leaving group. Leaving behind a carbocation intermediate, it is often necessary to consider possible carbocation rearrangements that would stabilize the positive charge.
In this case, no such rearrangement is favorable as their are no locations of greater stability available.
However, what must be considered is that the intermediate is free to orient itself in its most stable conformation prior to the formation of the double bond in the second step. As a result, the E product (the larger substituents are on oriented opposite one another with respect to the double bond) is yielded primarily.
Certified Tutor
All Organic Chemistry Resources














![\xrightarrow[]{LiAlH_{4}}\:?](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/463122/gif.latex)



