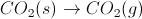All MCAT Physical Resources
Example Questions
Example Question #1061 : Mcat Physical Sciences
A metal object that is dropped into water. In which scenario would heat be transferred from the object to the water?
If heat is transferred from the metal object to the water, that means that the temperature of the metal must be greater than that of the water. We must find the answer choice where this is the case. Since the temperatures are given in different units, they must be converted to the same unit for comparison purposes.
Only one answer option results in the metal having a greater temperature than the water:
Example Question #1061 : Mcat Physical Sciences
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of 

What is the final temperature of the water and aluminum in the container?
315.5K
308.9K
301.6K
324.5K
301.6K
In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.

Example Question #52 : Physical Chemistry
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is 
What is the change in temperature as a result of the heating?
When a specific heat capacity is given, we typically use the equation 

Example Question #62 : Biochemistry, Organic Chemistry, And Other Concepts
Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.
Example Question #41 : Thermochemistry And Energetics
The formation of nitrous oxide is a 2-step process.
The overall enthalpy of the reaction is +68kJ.
If the enthalpy change of step 1 is 180kJ, what is the enthalpy change of step 2?
–112kJ
248kJ
112kJ
More information is needed to answer the question.
–112kJ
Hess's law states that the enthalpies of the steps in an overall reaction can be added in order to find the overall enthalpy. Since the overall enthalpy of the reaction is +68kJ, we can use the following equation to find step 2's enthalpy.
Example Question #54 : Physical Chemistry
Consider the following processes:
1)
2)
3)
Which for the following expresses 

1)
2)
3)
We need to add the given processes in such a way that leaves 

Since there are no 
First, reverse process 2 and multiply by two:
2a)
2a)
2a)
Add this result to process 1.
1)
2a)
1+2a)
Now we need to combine with process 3 to eliminate the 
3a)
3a)
Add this to the combined result from processes 1 and 2.
1+2a)
3a)
1+2a+3a)
This gives our final combination. The elemental reaction is 

Example Question #55 : Physical Chemistry
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
After the processes in each of the four scenarios above, what can be said about the entropy of the universe?
It increases in scenarios 3 and 4, only.
It increases in all scenarios.
It increases in scenarios 1 and 2, only.
It increases in scenarios 1 and 3, only.
It increases in scenarios 2 and 4, only.
It increases in scenarios 1 and 2, only.
Scenarios 3 and 4 are presented as thought experiments because, common sense tells us, they will never happen. The fundamental reason they will never happen is because they do not lead to a total increase in entropy of the universe. In order for an event to proceed, this requirement must be satisfied.
Example Question #1 : Entropy
Which of the following chemical or physical processes involves an increase in entropy?
Picking up a set of 
Entropy describes the number of configurations a system can have or, alternatively, how much "order" it possesses.
When water freezes, it forms a crystal lattice, which is more ordered than liquid water. Likewise, collecting playing cards and stacking them in a deck involves a transformation from disorder (cards scattered around the floor) to order (a single deck). Chemically, the precipitation of 



The disproportionation of 

Example Question #42 : Thermochemistry And Energetics
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
A scientist studying the dissociation of the Arrhenius base sodium hydroxide discovers that the reaction is very exothermic. What is true of the entropy change in the surroundings?
Assume the system refers to the reaction vesselcontaining aqueous sodium hydroxide.
The entropy of the surroundings is zero
The entropy of the surroundings increases
The entropy of the surroundings may increase or decrease
The entropy of the surroundings must remain constant
The entropy of the surroundings decreases
The entropy of the surroundings increases
The entropy change of the surroundings for an exothermic system must be positive. The release of heat by the exothermic reaction drives the increase in disorder of the environment surrounding the reaction vessel.
As the heat leaves the reaction system, it increases the entropy of the particles in the surroundings.
Example Question #43 : Thermochemistry And Energetics
Which of the following chemical processes involves a decrease in entropy?
Entropy decreases when the number of moles of gas decreases during a reaction. In the case of the correct answer, the number of moles of gas decreases from two to one.
When a substance goes from a solid to a gas (sublimation) or from a liquid to a gas (evaporation), entropy increases. Likewise, when a solid dissolves in water, entropy increases. Entropy (i.e. the number of arrangements a system can have) is much greater in a gas than in a liquid or solid. It is also greater for ions solvated in solution than for an un-dissolved solid.
Note that, in general, the entropy of the universe increases. In order for a process to involve a decrease in entropy of the system, there is likely a consequent increase in entropy of the universe.
Certified Tutor
Certified Tutor
All MCAT Physical Resources