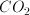All MCAT Physical Resources
Example Questions
Example Question #15 : Gas Laws
A gas with an initial volume of 




To solve this problem we use the combined gas law:
Use the given values for the temperature and volume, as well as the initial pressure, to solve for the final pressure.

Example Question #1 : Kinetic Molecular Theory
At room temperature which of the following equimolar gases has the fastest effusion rate?
According to Graham's law, the rate of effusion of a gas is inversely proportional to the square root of the mass: 
The gas with the lowest molecular weight will effuse the fastest. 

Example Question #481 : Mcat Physical Sciences
Consider a helium atom and a molecule of carbon monoxide both at a temperature of 
Since both molecules are at the same temperature, the value of the temperature is irrelevant. The difference in velocity between the two molecules depends on the difference in their molar masses. The molar mass of a helium atom is 

Graham's Law describes the relationship between gas particle mass and velocity:
Using the molar masses of the particles in question, we can determine their relationship.
Example Question #21 : Gases
Phase diagrams are used to depict changes in the properties of a solution at different temperatures and pressures. Below is a phase diagram of a polar solution.

In section 3, as the temperature increases, the velocity of the molecules __________ in a(n) __________ manner.
increases . . . indirectly proportional
decreases . . . indirectly proportional
increases . . . directly proportional
decreases . . . indirectly proportional
increases . . . indirectly proportional
This question asks you how the velocity of the molecules changes with increasing temperature. From molecular kinteics, we know that temperature is proportional to kinetic energy.
We can see that as temperature rises, the kinetic energy rises, however, the question asks us how the velocity changes. Now, we need to know how kinetic energy and velocity are related. We can pull this relationship from Newtonian mechanics.
If we simplify, we can see that 
Example Question #51 : Fluids And Gases
Under what conditions will a gas most closely follow ideal behavior?
High pressure and low temperature
Moderate levels of both temperature and pressure
Low pressure and high temperature
Low pressure and low temperature
High pressure and high temperature
Low pressure and high temperature
Ideal gases are assumed to have no intermolecular forces and to be composed of particles with no volume. Under high pressure, gas particles are forced closer together and intermolecular forces become a factor. In low temperatures intermolecular forces also increase, since molecules move more slowly, similar to what would occur in a liquid state. Just remember that ideal gas behavior is most closely approximated in conditions that favor gas formation in the first place—heat and low pressure.
Example Question #52 : Fluids And Gases
Which of the following assumptions is not made by the ideal gas law?
The van der Waals forces are negligible
The molecules move randomly
The intermolecular interactions follow the Coulomb model of electric repulsion
The size of the molecules is much smaller than the container
The molecules obey Newton's laws of motion at all times
The intermolecular interactions follow the Coulomb model of electric repulsion
Under the ideal gas law, we assume that the interactions between the molecules are very brief and that the forces involved are negligible. The assumption that the molecules obey Coulomb's law when interacting with each other is not necessary; rather, an ideal gas must disregard Coulomb's law.
The ideal gas law assumes only Newtonian mechanics, disregarding any intermolecular or electromagnetic forces.
Example Question #2 : Real Gases And Ideal Gases
Which of the following factors does not explain why measurements of real gases deviate from ideal values?
Intermolecular forces
The volume of the gas particles themselves
The volume between the gas particles
All of these factors will cause deviation from ideal values
The volume between the gas particles
Measurements of real gases deviate from ideal gas predictions because intermolecular forces and the volume of the particles themselves are not taken into consideration for ideal gases. The volume of the space between particles is considered for ideal gases and does not contribute to deviation from ideal gas behavior.
Attraction between molecules causes real pressure to be slightly less than ideal pressure, while the volume of gas particles causes real volume to be slightly greater than ideal volume.
Example Question #3 : Real Gases And Ideal Gases
The definition of ideal pressure for a real gas is given as follows:
The constant 
Which of the following would have a negative attraction coefficient, 
Dichloromethane
Magnesium with an electron configuration of
Sodium chloride
Oxygen with an electron configuration of
Magnesium with an electron configuration of
The question wants you to pick a molecule that will have a negative attraction coefficient, 

Dichloromethane 


On the other hand, magnesium, with an electron configuration of 




Example Question #2 : Real Gases And Ideal Gases
Consider a real gas with a constant amount and a constant pressure. It has a temperature of 

The volume will become
The volume will become less than
The volume will become
The volume will become greater than
The volume will become less than
This question can be solved using either Charles's law or the ideal gas law (converted into the combined gas law).
Charles's Law:
Ideal Gas Law:
The question states that the pressure and moles 
The volume would double for an ideal gas; however, the question is asking about a real gas. To find the correct relationship between volume and temperature we need to look at the equation for real gas volume. Remember that the volume we are concerned with is the volume of the free space in the container, given by the container volume minus the volume of the gas particles. The equation for real gas volume accounts for the volume of the container and the volume of the gas particles. For a real gas, the volume is given as follows:
In this equation, 

Note that for an ideal gas the bigness coefficient, 


Example Question #53 : Fluids And Gases
Which of the following gases would behave the least ideally?
For a gas to behave ideally, is should have a low mass and/or weak intermolecular forces. Contrastingly, non-ideal gasses should have very large masses and/or have strong intermolecular forces. Therefore, the correct answer is 
Certified Tutor
Certified Tutor
All MCAT Physical Resources