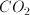All MCAT Physical Resources
Example Questions
Example Question #11 : Gases
Regarding the following sets of conditions, which answer option gives the correct listing of systemic pressures from greatest to least?
This question asks for you to look at a set of conditions for gases, and determine relative pressures. The best equation to use for quick calculation and relation is the ideal gas law, given by:
Rearranging this, and removing the constant (since it will not affect relative pressure), we can generate a proportionality of pressure to the other variables.
We can use this proportionality with each option to determine their rankings by pressure.
Example Question #41 : Fluids And Gases
How many grams of ammonia gas are in a 


First, convert temperature to Kelvin.
Next, use the ideal gas law to solve for moles.
Finally, convert moles of ammonia to grams using molar mass.
Example Question #11 : Gases
What is the temperature of a 1L container at STP after the pressure is doubled?
546K
298K
273K
596K
200K
546K
Using Gay-Lussac's Law, which is 
Because the problem states that the original conditions were at STP, we know that pressure is 1atm and temperature is 273K. Since pressure and temperature are directly proportional, doubling pressure will also double the temperature. The final temperature will be 546K.
Example Question #42 : Fluids And Gases



Remember to convert temperature to Kelvin when using the gas equations.
We will use Charles's Law to calculate the new volume:
Use the given temperatures and initial volume to calculate the final volume.
Example Question #11 : Gas Laws
Which of the following would not cause a decrease in the pressure of a gas in a sealed container?
Increasing the volume of the container
Adding moles of a different gas
Reducing the temperature
Removing gas particles from the container
Adding moles of a different gas
A decrease in pressure means a decrease in gas particle collisions. The only option that would not cause a decrease in collisions is adding moles of a different gas. Even though different molecules are added, there will be greater pressure as particle collisions will be more frequent.
Reducing temperature slows the gas particles, thus decreasing the frequency of collisions. Similarly, increasing the volume of the container and removing particles will cause a decrease in collisions, and subsequent pressure.
Example Question #12 : Gases
A sealed container holds three moles of gas at 1atm and 200K. Its pressure is to 2atm. What will be the resulting temperature in the container?
300K
400K
273K
100K
250K
400K
In the problem, the volume and the number of moles are constant and the temperature and pressure are the only two variables that are changing. Using the ideal gas law we can find that temperature and pressure are directly proportional. When pressure increases by a factor of two, temperature will also increase by a factor of two.
Example Question #13 : Gases
A 




The amount of gas is irrelevant. If the temperature is held constant and the volume is increased by a factor of three, the resulting pressure is decreased by a factor of three according to Boyle’s Law.
Example Question #15 : Gas Laws
A gas with an initial volume of 




To solve this problem we use the combined gas law:
Use the given values for the temperature and volume, as well as the initial pressure, to solve for the final pressure.

Example Question #51 : Fluids And Gases
At room temperature which of the following equimolar gases has the fastest effusion rate?
According to Graham's law, the rate of effusion of a gas is inversely proportional to the square root of the mass: 
The gas with the lowest molecular weight will effuse the fastest. 

Example Question #481 : Mcat Physical Sciences
Consider a helium atom and a molecule of carbon monoxide both at a temperature of 
Since both molecules are at the same temperature, the value of the temperature is irrelevant. The difference in velocity between the two molecules depends on the difference in their molar masses. The molar mass of a helium atom is 

Graham's Law describes the relationship between gas particle mass and velocity:
Using the molar masses of the particles in question, we can determine their relationship.
Certified Tutor
All MCAT Physical Resources