All MCAT Biology Resources
Example Questions
Example Question #41 : Reactions
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Reaction 1 proceeds more quickly, owing to a more stable carbocation
Reaction 1 proceeds more slowly, owing to a less stable carbocation
Reaction 1 only proceeds with a stronger nucleophile
Reaction 1 proceeds more quickly, owing to a decrease in steric hindrance
Reaction 1 proceeds more slowly, owing to a higher activation energy
Reaction 1 proceeds more slowly, owing to a higher activation energy
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Example Question #13 : Reaction Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
IV, only
I and II
II, only
III, only
I, only
IV, only
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Example Question #503 : Organic Chemistry

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?

None of these




The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

Example Question #1452 : Mcat Biological Sciences
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Reaction rate in this reaction is not determined by concentration
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Example Question #13 : Reaction Mechanisms
When exposed to a good nucleophile, which molecule will most readily undergo an 

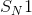

Example Question #11 : Reaction Mechanisms
Which of the following compounds can be oxidized to form a ketone?
2-methyl-2-butanol
3-pentanol
Methanol
Propyl alcohol
3-pentanol
An alcohol can be oxidized and form a ketone only if the alcohol is a secondary alcohol. Tertiary alcohols cannot be oxidized and primary alcohols are oxidized to form aldehydes and carboxylic acids.
3-pentanol is a secondary alcohol, while 2-methyl-2-butanol is a tertiary alcohol. Methanol and propyl alcohol are both primary alcohols.
Example Question #1453 : Mcat Biological Sciences
What reagent would be best suited to accomplish the transformation shown below?

Methyl magnesium bromide,
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

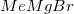
Example Question #1 : Other Reaction Mechanisms
The transformation of compound B to compound C below is known as what type of reaction?

Hydration
Hydroboration
Decarboxylation
Hydrogenation
Dehydration
Dehydration
The conversion of compound B to compound C results in the elimination of water, which, by definition, is a dehydration reaction. The hydroxyl group on compound B is protonated by the sulfuric acid, generating an 
Hydroboration is the oxidation of an alkene with a borohydride (usually sodium borohydride) reagent, to produce an alkane. Hydration involves the use of a water reactant, usually producing an alcohol product. Hydrogenation is the oxidation of alkene double bonds with the use of a palladium interface to produce an alkane product. Decarboxylation results in product carbon dioxide from a carboxylic acid or carbonic anhydrase reactant.
Example Question #2 : Other Reaction Mechanisms
Which alcohol will react most rapidly via an SN1 mechanism?
Tertiary alcohols react most rapidly via SN1 mechanisms because they form stable tertiary carbocations. Primary and secondary alcohols typically react most rapidly via SN2 mechanisms.
Of the available options, 
Example Question #371 : Organic Chemistry, Biochemistry, And Metabolism
What type of enzymatic inhibitor binds to an allosteric location on the enzyme with equal affinity for the bound and unbound substrate states?
Competitive inhibitor
Suicide inhibitor
Uncompetitive inhibitor
Noncompetitive inhibitor
Noncompetitive inhibitor
Noncompetitive inhibitors are a specific type of mixed inhibitor that binds to both the free enzyme and the enzyme-substrate complex with equal affinities, resulting in the same binding affinity (Km value) but a decrease in the maximum rate of reaction (Vmax value).
Certified Tutor
All MCAT Biology Resources


















