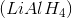All MCAT Biology Resources
Example Questions
Example Question #1 : Other Reactions
Which of these methods can be used to synthesize a secondary alcohol?
Reacting propene with water and acid
Reacting a ketone with lithium aluminum hydride, then adding water
Adding a Grignard reagent to an aldehyde, then reacting with acid
All of these answer choices are correct
All of these answer choices are correct
All of these methods can successfully synthesize secondary alcohols.
Lithium aluminum hydride and sodium borohydride are strong and weaker reducing agents, respectively. Both are able to reduce ketones to secondary alcohols.
Adding water and acid to an alkene (such as propene) results in Markovnikov addition of a hydroxyl group, also creating a secondary alcohol.
Grignard reagents (organometallic halides) add to the carbonyl carbon of an aldehyde, adding an alkane group and forming an alcohol product.
Example Question #1 : Other Reactions
Which of the following is an example of an anabolic reaction in the body?
Condensation reactions
Hydrolysis
Decomposition reactions
The conversion of glycogen to glucose
Condensation reactions
Anabolic reactions are reactions that construct molecules from smaller primary units. Catabolism is just the opposite, where larger molecules are degraded into simpler subunits. In the body larger macromolecules, such as proteins, are frequently created via condensation reactions. In condensation reactions, water is formed as a byproduct of joining two molecules together.
All other three processes describe a scenarios where a larger molecule is broken down into smaller subunits, known as catabolic processes.
Example Question #2 : Other Reactions
In the human body, glycerol and three fatty acids can act together to form triacyl glycerol in a process known as esterification. The reverse of this process is known as __________.
reverse esterification
transesterification
decarboxylation
saponification
saponification
When an alcohol and carboxylic acid are reacted together they can form an ester, in a process known as esterification. This is what happens with glycerol and fatty acids to form triacyl glycerol. The reverse of this process is an organic chemistry reaction known as saponification, in which an ester and water are reacted to form carboxylic acids and alcohol.
Example Question #21 : Reactions
Triacylglycerols are broken down in the body by lipases. In the presence of water, lipase is used to break down the triacylglycerol into a glycerol molecule and three fatty acids. Based on this information, the breakdown of a triacylglycerol is the opposite of which reaction?
Esterification
Hydrolysis
Decarboxylation
Saponification
Esterification
The molecular structure of a triacylglycerol reveals that the three fatty acid tails are attached to the glycerol molecule, resulting in three ester functional groups. Remember that fatty acids have a carboxylic acid functional group at one end, which is created after the cleavage described in the question. Since the ester bonds are broken and carboxylic acids are created following the hydrolysis of a triglyceride, the opposite of esterification has taken place.
The process described (breakdown of an ester into a carboxylic acid) is also known as saponification.
Example Question #1 : Help With Oxidation Reduction Reactions
Which of the following is not capable of oxidizing a secondary alcohol to a ketone?
Pyridinium chlorochromate (PCC)
Lithium aluminum hydride


All of these answers can oxidize secondary alcohols to ketones
Lithium aluminum hydride
Lithium aluminum hydride is correct because it is a reducing agent, and is therefore not capable of oxidizing secondary alcohols. Instead, LAH could be used to perform the reverse reaction, reducing a ketone to an alcohol. The other answer choices are oxidizing agents.
Example Question #49 : Organic Chemistry, Biochemistry, And Metabolism
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
A patient overdosed on an unknown drug. Two hours after being admitted into the hospital, acetazolamide was administered. A realtime measurement of the drug's concentration in the patient's blood was taken the moment the patient was admitted every 30 minutes. Which of the following best explains the lab results shown?

The unknown drug was acidic
The unknown drug was basic
The unknown drug destroyed the renal system
The unknown drug was innate
The sample was influenced by the body's metabolic
The unknown drug was basic
From the data, the concentration of the unknown drug in the blood increased shortly after the patient received the carbonic anhydrase inhibitor. The increase in the blood's concentration of the drug meant that the body is reabsorbing the drug. The only way for the drug to be reabsorbed is if it is able to cross the membrane of the renal's lumen. In order to cross the renal's membrane, the drug must be noncharged and nonpolar. Basic molecules in a basic environment will stay uncharged.
Example Question #50 : Organic Chemistry, Biochemistry, And Metabolism
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
Glaucoma involves the pressure within the eye increasing to a dangerously high level due to improper draining of the vitreous humor. Blindness usually follows due to damage to the nerves. How might administering a carbonic anhydrase inhibitor help in patients with glaucoma?
Increase intraocular pressure
Prevent the excess production of fluid
Increase the production of fluid
Increase acid production
Lower the rate of vitreous humor drainage
Prevent the excess production of fluid
From the equation:
We can see that carbonic anhydrase functions in both the forward and backward reactions. In the eye, the equation shifts to the left and more water is produced. Water is one of the main substances in vitreous humor. As the production of water increases, pressure increases as well. By blocking the production of water, the pressure is lowered.
Example Question #26 : Reactions
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
When a patient is not ventilating efficiently, the kidneys try to compensate by producing more bicarbonate to buffer the decreasing pH of the blood due to the buildup of 

I. Increase 
II. Increase 
III. Increase metabolism
II only
I, II, and III
III only
I and III
I only
I and III
To answer this question, we must understand Le Chatelier’s principle. The reaction favorites the product when there is more of the reactants. The opposite is also true in that when there is a buildup of the products, the reaction will reverse and favorite the reactants. Increasing the metabolism will produce more 
Example Question #11 : Other Reactions
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
Based on the passage, which of the following statements, if true, will contradict the effectiveness of carbonic anhydrase inhibitors as a treatment?
Acidic molecules will not release its proton in the basic environment of the lumen of the collecting tubule
Acidic molecules will release its proton in the basic environment of the lumen of the collecting tubule
Acidic molecules have a better ability to cross the membrane than basic molecules
Basic molecules will release its proton in the basic environment of the lumen of the collecting tubule
Basic Molecules will not release its proton in the basic environment of the lumen of the collecting tubule.
Acidic molecules will not release its proton in the basic environment of the lumen of the collecting tubule
Carbonic anhydrase inhibitors are used to alkalinize the urine. When the urine is alkalinized, acidic molecules will lose its proton and go into a charged state. Charged molecules are unable to cross the membrane of the lumen of the collecting tubule. Without the ability to cross the membrane, the molecule is therefore unable to be reabsorbed. Therefore, if the statement "Acidic molecules will not release its proton in the basic environment of the lumen of the collecting tubule" was true, then alkalinizing the urine will have no effect.
Example Question #1 : Substitution And Elimination Mechanisms
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
SN1
SN2
E2
E1
E2
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Certified Tutor
All MCAT Biology Resources