All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Oxidation Reduction Reaction
Which substance is used as a reducing agent?
A reducing agent is a substance that readily donates an electron to another substance. They consist primarily of elements with low electronegativities such as hydrogen. Therefore, the answer is 
Example Question #1 : Intermediates
What intermediate is involved in the conversion of compound B to compound C?

Tertiary carbanion
Tertiary radical
Secondary carbocation
Secondary radical
Tertiary carbocation
Tertiary carbocation
The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
Example Question #1 : Properties Of Hydrocarbons
Which of the following carbocation intermediates requires the least activation energy?
Cannot be determined





The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Example Question #44 : Organic Chemistry
Carbon 1:

Carbon 2:

Let's say we react the given compound with 
Carbon 2 because the bromine will first attack the double bond from the least sterically hindered side
Carbon 1 because the intermediate will be a primary carbocation
Carbon 1 because the bromine anion is large and requires space to react
Carbon 2 because the intermediate will be a secondary carbocation
Carbon 1 because the intermediate will then be a secondary carbocation
Carbon 1 because the intermediate will then be a secondary carbocation
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of 




If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
Example Question #1 : Intermediates

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?
No because secondary carbocations are unstable
No because bromine is an electrophile
Yes because bromine donates some electrons
No because bromine's bulky electron cloud will interfere
Yes because bromine is an electrophile
No because bromine is an electrophile
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
Example Question #3 : Intermediates
Which of the following steps of free radical chlorination does not produce a free radical as a product?
Halogenation
Termination
Propagation
Initiation
Termination
The three steps of a free radical chlorination reaction are, in order, initiation, propagation, and termination.
Free radicals are produced in the initiation and propagation steps. The termination steps combine any two free radicals formed in the reaction to produce a compound that has no unpaired electrons (free radicals).
Example Question #2 : Intermediates
Which of the following transformations includes an enolate intermediate?
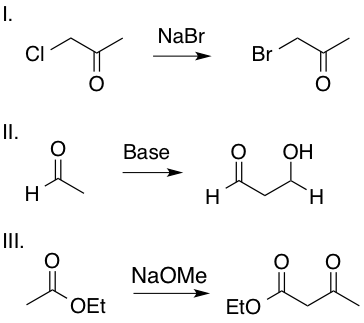
I and III
II and III
I, II, and III
III only
I and II
II and III
Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.

Example Question #1503 : Mcat Biological Sciences
The molecules shown below are best described as __________.
enantiomers
diastereomers
epimers
isomers
isomers
The molecules in this problem are isomers because they each have unique configurations and do not share the same funcitonal groups at the same carbon positions. Enantiomers are reflections of each other. Diastereomers are stereoisomers that differ at one or more stereocenters, while epimers are stereoisomers that differ at only one stereocenter.
Example Question #1 : Isomers
Which of the following is not a geometric isomer of pentene?


All of these are geometric isomers of pentene.



Geometric isomers are compounds that have the same molecular formula but they differ in the way they are arranged spatially. Pentene carries the molecular formula, 

 and this compound is not a geometric isomer of pentene.
and this compound is not a geometric isomer of pentene.
Example Question #1501 : Mcat Biological Sciences
A molecule has three chiral centers. How many stereoisomers of this compound will have different boiling points compared to the original molecule?
Two
Six
Seven
One
Six
The first step is to determine how many stereoisomers there are for this molecule. Since the number of stereoisomers is dependent on the number of chiral carbons, we can solve according to the equation 

Next, we need to compare the different stereoisomers to the original molecule. The original molecule will have one enantiomer and six diastereomers. Remember that enantiomers have the same physical properties, so we will not include this isomer in the final answer. Diastereomers, on the other hand, have different physical properties compared to the original molecule. As a result, six stereoisomers will have different boiling points compared to the original molecule.
All GRE Subject Test: Chemistry Resources