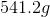All College Chemistry Resources
Example Questions
Example Question #1 : Stoichiometric Calculations And Dimensional Analysis
Consider the unbalanced equation for the combustion of pentane:
How many grams of 


Start by balancing the given chemical equation:
Next, convert the grams of pentane into moles of pentane.
Use the stoichiometric ratio given by the balanced equation to find the number of moles of oxygen needed to react with the pentane.
Finally, convert the number of moles of oxygen into grams of oxygen.
There should be 

Example Question #2 : Stoichiometric Calculations And Dimensional Analysis
Consider the following unbalanced reaction:
How many grams of 




Start by balancing the equation:
Next, figure out which reactant is the limiting reactant.
Since fewer moles of 


Convert the amount of moles of 
Example Question #7 : General Topics
What is the mass in 


For this question, we're given the density and volume of a gas and we're asked to find the mass of the gas.
To answer this question, we'll need to use dimensional analysis. What this means is that we'll need to cancel out units in order to obtain the ones that we're looking for.
Example Question #31 : Reactions
Use the equation shown to determine how many grams of water form when 








Use the relations 

All College Chemistry Resources