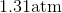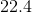All College Chemistry Resources
Example Questions
Example Question #1 : Non Ideal Gas Behavior
One flask is at STP and another is at 
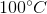
The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Example Question #1 : Non Ideal Gas Behavior
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.

Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Example Question #1 : Gas Laws
What is the pressure of a 


Recall the ideal gas law:

where 



and 
Since the question asks for pressure, rearrange the equation to solve for 
Plug in the given values. Remember that
Example Question #2 : Gas Laws
A sample of gas at a constant temperature has an initial pressure of 


Since we are given the volume and the pressure of this sample of gas, we will need to use Boyle's Law, which states that the pressure and volume of a gas, at a constant temperature, are inversely related. As thus, we can then write the following equation:
Since all the answer choices are in units of atmospheres, we will need to convert the given units into atmospheres.
Plug in the given pressures and volume into the equation, and solve for 
Example Question #3 : Gases
Consider the following chemical reaction:
How many grams of lithium is needed to react with 


Start by finding the number of moles of nitrogen gas by using the ideal gas law:
Rearrange the equation to solve for the variable 
In order to use the ideal gas law, the pressure must have units of atmospheres and the temperature must have units of Kelvin.
Convert the given pressure into atmospheres:
Now, substitute in all the given values in order to find the number of moles of nitrogen gas.
Next, use the stoichiometric ratio given by the chemical equation to find the number of moles of lithium needed to react completely with the nitrogen gas.
Finally, convert the number of moles of lithium to number of grams of lithium.
Example Question #1 : Gases
What is the pressure exerted by 


Example Question #1 : Gas Laws
Avogadro's Laws relate which of the following variables?
Volume is inversely proportional to pressure
Volume is proportional to temperature
Volume is proportional to moles of gas
Volume is inversely proportional to temperature
Temperature is proportional to moles of gas
Volume is proportional to moles of gas
Avogadro's law states that volume is proportional to moles of gas. Temperature and pressure are fixed. Boyle law states that volume of gas is inversely proportional to pressure. Charles' law states that volume of gas is proportional to temperature.
Example Question #1 : Gas Laws
What is the volume occupied by 


Recall the Ideal Gas Law:
Since the question is asking for volume, rearrange the equation to the following:
Also recall that for the Ideal Gas Law equation to work, the temperature must be given in degrees Kelvin, and we will also need the number of moles of gas.
Start by converting the mass of gas into moles:
Don't round the values just yet.
Next, convert the temperature into degrees Kelvin.
Now, plug in all the given values into the equation to solve for volume:
The answer should have 
The volume is 
Example Question #11 : Gases
A cylinder contains 


Recall the Ideal Gas Law:
Because we want the amount of gas in the cylinder, we must find the number of moles of oxygen present first. Rearrange the Ideal Gas Law to solve for the number of moles of oxygen.
Plug in the given information to solve for the number of moles.
Now, convert the number of moles to the number of grams by using the molar mass of oxygen gas.
Make sure that your answer has 
Example Question #5 : Gas Laws
Ideal gas law calculation
The given equation shows the synthesis of methanol. Find the volume in liters of 



Use the ideal gas equation
Rearrange to solve for the volume
All College Chemistry Resources

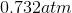
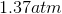

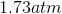



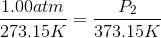


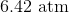




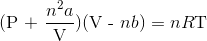
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)