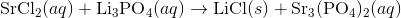All College Chemistry Resources
Example Questions
Example Question #1 : Vaporization And Condensation
Suppose a source of water is boiling. If the ambient pressure above the surface of the water were to increase, which of the following would happen to the boiling water?
The water would solidify into ice
The water would stop boiling at first, but then would continue to boil
The water would stop boiling
The water would continue to boil
The water would continue to boil at a faster rate
The water would stop boiling
For this question, we're told that water is boiling and that the pressure above the surface of the water increases. We're asked to find out what will happen to the water.
It's important to remember the definition of a boiling liquid; the vapor pressure of the liquid is equal to the atmospheric pressure above the solution. Thus, if the atmospheric pressure increases, the vapor pressure would then become lower than the atmospheric pressure. As a result, the water would cease to boil. Moreover, its boiling point would increase because a higher temperature would be necessary in order to raise the vapor pressure enough so that it becomes equal to atmospheric pressure.
Example Question #1 : Freezing And Melting
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
Thermal equilibrium: Thermal energy of the system is equal to thermal energy of the surroundings.
Thermal equilibrium: specific heat capacity of ice is greater than water and the ice melts quickly.
Work: the system did work on the surroundings and lost heat, resulting in a colder glass of water with condensation
Energy: potential energy of the ice was converted to kinetic energy of the water.
Chemical Potential Energy: intermolecular forces of the water broke apart the intramolecular forces within the ice and absorbed the excess energy.
Thermal equilibrium: Thermal energy of the system is equal to thermal energy of the surroundings.
Thermal equilibrium: Thermal energy of the system is equal to thermal energy of the surroundings. Heat is defined as the energy transfer resulting from differences in thermal energy. Heat always flows from higher temperature to lower temperature. Heat transfer between a system and its surroundings stop when they reach thermal equilibrium, or when there is no difference in thermal energy. In this case, ice was dropped into the cup. Initially, the ice increases the temperature of the water, creating a cold glass with condensation. However, energy from the surroundings flow into the system (the glass of water) due to thermal difference and warm the glass of water until the two reach thermal equilibrium. At thermal equilibrium, there is no ice, no condensation, and the water temperature is room temperature.
Example Question #1 : Freezing And Melting
Suppose that 


Note: Liquid water has a density of 

In this question, we're told that a certain amount of a compound dissolved in water lowers the water's freezing point. We're asked to determine the molar mass of the compound.
First, we must recognize this as a freezing point depression problem. Recall that freezing point depression is one of the colligative properties associated with dissolving solute into a solvent such as water.
To begin, we need to use the equation for freezing point depression, which states that the change in freezing point is proportional to both the molality of the solution as well as the molal freezing point depression constant for the solvent in question (in this case, water).
The answer we have just calculated above is the molality, which is a different way of expressing concentration than molarity. Molality is expressed as the number of moles of solute per kilogram of solvent, whereas molarity is the number of moles of solute divided by the total volume of the solution.
The next thing we need to do is find out how many total moles of solute are present in the solvent water. To do this, we need to use the volume of water provided to us in the question, along with the density of water, to calculate the mass of water present. Together with the molality calculated above, this will allow us to know the total number of moles in solution.
Now that we know the total number of moles of solute (our compound) that exists in solution, we can use that information, together with the total mass of the compound given to us in the question stem, to calculate the compound's molar mass.
Example Question #2 : Freezing And Melting
Ice can be used to counter the effects of overeating. How many kilograms of ice at 


Specific heat of water =
Specific heat of ice =
Recall that the heat gained by ice must be equal to the heat from the food.
Start by calculating the heat from the food by converting the Calories into joules.
Next, calculate the heat gained by the ice. Take this in three steps:
1. Heating the ice from 
2. Ice melting.
3. Raising the temperature of water from 

Set this value equal to the heat from the food and solve for the mass.
Convert to kilograms.
Example Question #11 : Solutions, States Of Matter, And Thermochemistry
As heat energy is added to a cube of ice, it begins to melt into liquid water. Which of the following correctly identifies the change in temperature and the change in internal energy of the ice as heat is added to it?
The temperature and the internal energy both remain constant
The temperature increases while the internal energy remains constant
The temperature remains constant while the internal energy increases
The temperature and the internal energy both increase
The temperature remains constant while the internal energy increases
Remember that when any substance is undergoing a phase change, its temperature will remain constant. In other words, the energy being added is not increasing the average kinetic energy of any of the particles in the system.
Also, the internal energy will not remain constant. As heat energy is added, as in the question, the internal energy will necessarily increase. Even though the kinetic energy of the particles is not increasing, the potential energy is. This is because the intermolecular forces of attraction (mostly hydrogen bonds in this case) need to be broken apart. When energy is added, that raises the potential energy component of internal energy, despite the fact that kinetic energy (and thus, temperature) remains constant.
Example Question #12 : Solutions, States Of Matter, And Thermochemistry
If a liquid has a low resistance to flow, it has a low __________.
Melting point
Viscosity
Volume
Boiling point
Vapor pressure
Viscosity
By definition, viscosity is the measure of a liquid's resistance to flow. If a liquid has a low viscosity, it has a low resistance to flow. It may not necessarily always have a low boiling point, melting point, and/or vapor pressure due to this characteristic. Viscosity is just a way to measure and describe one physical characteristic (resistance to flow) of a liquid. The volume of a liquid does not say anything about its physical properties.
Example Question #42 : College Chemistry
Write an equation for the precipitation reaction that occurs (if it occurs) when a solution of strontium chloride is mixed with a solution of lithium phosphate.
Start by writing out the the chemical formulas for the reactants:
In order to figure out the possible products, combine the cation of one molecule with the anion of the other molecule.
For this reaction, the possible products are 

Next, use solubility rules to figure out if any precipitate is formed. Since compounds with 




Thus, we can write the final chemical equation:
Example Question #3 : Liquids And Solutions
Which of the following is insoluble in water?
Recall the solubility rules:


Example Question #4 : Liquids And Solutions
Which of the following substances is insoluble in aqueous solution?
Solubility rules can help us find the answer to this question.
First, solubility rules state that all compounds of Group 1A elements on the periodic table are soluble in aqueous solution. This means that all of the alkali metals, including potassium, form compounds which are soluble in aqueous solution; thus, 
Solubility rules also tell us that all ammonium salts (salts of 

Next, solubility rules tell us that all bromide salts are soluble, except for those of 




Example Question #1 : Liquids And Solutions
Which of the following is not readily soluble in water?
Remember the solubility rules for ionic solids in water:
1) Salts of group 1 (with few exceptions) and NH4+ are soluble
2) Nitrates, acetates, and perchlorates are soluble
3) Salts of silver, lead, and mercury (I) are insoluble
4) Chlorides, iodides, and bromides are soluble
5) Carbonates, phosphates, sulfides, oxides, and hydroxides are insoluble. Exceptions: sulfides of group 2 cations and hydroxides of calcium, strontium, and barium are slightly soluble
6) Sulfates are soluble except for those of calcium, strontium, and barium
Following these rules, we see that MgOH is insoluble in water
All College Chemistry Resources