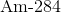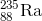All College Chemistry Resources
Example Questions
Example Question #1 : Atoms And Elements
Isotopes differ in the number of __________.
covalent bonds
electrons
protons
lone pairs
neutrons
neutrons
By definition, isotopes of a given element have the same number of protons and electrons, but differ in the number of neutrons. This causes a difference in the mass number (protons + neutrons) as well. Neither the number of protons nor the number of electrons changes with different isotopes of the same element.
Example Question #1 : Isotopes
Which of the following is not an isotope of hydrogen?
Deuterium
Tritium
Protium
Quatrium
Quatrium
Isotopes are versions of an element with different numbers of neutrons. Hydrogen has three naturally occurring isotopes. 


Example Question #3 : Atoms And Elements
Chromium has four naturally occurring isotopes. 











Using this information, estimate the atomic mass of chromium, in atomic mass units.
In order to find the molar mass of an atom from its isotopes and their natural abundances, use the following equation:

Since chromium has four isotopes, we will write the following equation to find its atomic mass:
Example Question #1 : Isotopes
Isotopes of a given element differ in the number of __________.
Electrons
Protons
Photons
Neutrons
Neutrons
Each element is defined by the number of protons its atoms contain. For example, hydrogen has one proton, helium has two protons, and lithium has three protons. Each element also has a characteristic number of neutrons. For example, hydrogen has zero neutrons, helium has two neutrons, and lithium has four neutrons.
Some elements, however, also have different "versions" of themselves: atoms which have a different number of neutrons, called isotopes. For example, there are three isotopes of hydrogen. 


Example Question #2 : Atoms And Elements
What is the daughter nuclide when 
Recall that when a particle undergoes alpha decay, the particle is emitting an alpha particle, which is the same as 
Now, write the following equation of the alpha decay:
Thus, 
Example Question #261 : College Chemistry
If 

For this question, we're told that 

The first thing we need to do is write an expression for radioactive decay. Remembering that radioactive decay processes follow a first-order reaction rate, we can use the following expression.
We can further rewrite this expression as follows.
Also, recall that in the beginning we are starting with 




Now that we have the rate constant for the decay reaction, we're equipped with what we need in order to calculate the half-life.
To solve for the half-life, we can derive an expression using one already shown above.
Now, using the above expression, we can plug in the value for the rate constant that we calculated in order to solve for the half-life.
Example Question #261 : College Chemistry
What is the daughter nuclide when 
Recall that beta decay occurs when the nucleus of the parent atom emits an electron. We can then write the following equation to illustrate the beta decay of thorium.
Make sure that the atomic numbers and masses add up to the same on both sides of the equation.
Example Question #263 : College Chemistry


In order to solve this problem we first must setup an equation representing the decay of 


where 

and 
Let's start with the atomic number:
Since we have an atomic number of 



As an equation this is written as:
This simplifies to 


Now we must solve for the mass number similarly:
Following the instructions above except for the mass number we create an equation with one unknown to solve for the mass number of 
therefore
This means our final answer is 
This can be double-checked by plugging the mass number and atomic number into the decay equation and simplifying it.
Example Question #264 : College Chemistry
Which of the following sequences describe the decay process from 

Beta, gamma, alpha, alpha
Alpha, beta
Beta, beta
Alpha, alpha, beta
Beta, beta, beta
Beta, beta, beta
In order to determine the decay process that occurs we should check if the mass number charges or not in the decay. Since the mass number doesn't change we can eliminate any answer with 
Now we can write out our decay equation. Since the atomic numbers aren't written in the question we must find them on the periodic table, where elements are ordered by their atomic number. In the case of Thallium, we find that it is element number 

So our equation is as follows:
where 

and 
Since the mass number is the same on both sides of the equation we know that it is equal to 
Now we see that the atomic number increases by 






Example Question #265 : College Chemistry

Positron emission
Alpha (
Electron capture
Beta (
Gamma emission (
Alpha (
Let's first look at the cases and determine which ones can maintain both the same atomic and mass number. We know that 


As for 

In electron capture, an electron is gained so the atomic number decreases, so this cannot be the right answer either.
Positron emission means a positron 
Therefore the right answer is gamma 
Certified Tutor
All College Chemistry Resources