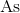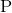All College Chemistry Resources
Example Questions
Example Question #266 : College Chemistry
When a 


In order to begin determination of the mass number of phosphorus, we must first write out the equation and then solve for the mass number of phosphorus.
We know there is 












Now let's write out the reaction that occurs and all of its reactants and products, so we can solve for X:

Now we can solve for the unknown mass number of phosphorus by adding up the mass numbers on each side of the equation and solving for the unknown.
On the reactants side we have 



This leaves us:
Therefore the mass number of phosphorus is 
Example Question #267 : College Chemistry

In an electron capture, an electron is absorbed meaning the atomic number of the product is reduced by 1. Therefore we can write out our radioactive decay equation as:



Where 

and 
Since the mass number doesn't change at all 





Example Question #268 : College Chemistry


In 



Where 

and 
We can quickly determine the atomic number/element name by making an equation with an unknown with all the atomic numbers. This would be:
therefore the atomic number is 
We can do the same for mass numbers to get the equation:


Example Question #269 : College Chemistry
What is the daughter nuclide after 
Recall what an alpha particle is: 
Now, write the equation for the alpha decay of polonium-214. The polonium will be emitting an alpha particle.
Make sure that the masses and the atomic numbers on both sides of the equation will add up.
Example Question #71 : Introductory Topics
This is a question regarding radioactive decay, specifically alpha decay. An alpha particle has the same identity as the nucleus of a 








Example Question #81 : Introductory Topics
Which of the following elements has the most metallic character: 
The metallic character of elements increases as you move down a group. All of the listed elements are in Group 5. Since 
Example Question #11 : Atoms And Elements
Which of the following elements has the largest atomic radius: 
When looking at a periodic table, you should notice that all of these elements are in the same period. Recall that atomic radii decrease as you move right on a period. Thus, this is the order of the elements by decreasing radius:

Example Question #3 : Periodic Trends
The set of elements with the highest first ionization energies are known as which of the following?
Noble gases
Halogens
Alkaline earth metals
Metalloids
Alkali metals
Noble gases
The noble gases possess a full octet of electrons. They have the largest first ionization energies because of their tendency to keep all of their valence electrons. Halogens have the highest electron affinity; therefore, they have high—but not the highest—ionization energies. The alkali and alkaline earth metals have low electron affinities and low ionization energies. Last, the metalloids possess ionization energies that are neither high nor low.
Example Question #1 : Periodic Trends
Rank the following in order of increasing metallic character:
The correct answer is




Example Question #4 : Periodic Trends
Which of these atoms is the smallest?
Beryllium
Magnesium
Barium
Strontium
Calcium
Beryllium
The atomic radii is the size of an atom when it is not bonded to any other atoms. The periodic table can be used to estimate the size of the atomic radii of atoms. As you move down the periodic table the atomic radii increase, but as you move from left to right on the periodic table, the size of the atomic radii decrease.
Beryllium, magnesium, calcium, strontium, and barium are all in the same group on the periodic table, so the smallest element is the element closest to the top of the periodic table since the atoms become larger as you go down the periodic table. Therefore, the smallest atom of this group is beryllium.
All College Chemistry Resources