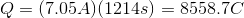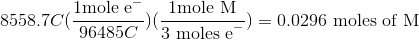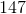All College Chemistry Resources
Example Questions
Example Question #1 : Reactions
Electrolysis of an unknown metal chloride, 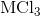
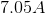
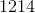
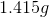
Titanium
Scandium
Chromium
Nickel
Titanium
Start by writing out the equation that illustrates the plating out of the metal:
Note the stoichiometric ratio for the moles of electrons to the moles of the metal.
Next, recall the following equation:
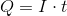



Plug in the given information to find 
Next, recall Faraday's law of electrolysis that equates Faraday's constant and the amount of electrons together to find the charge needed to deposit one mole of a particular substance.
Thus, we can set up the following to take the charge of the electrolysis through to figure out the number of moles unknown metal that was plated out.
Now, since we have the number of grams of the metal deposited, we can find the molar mass of the unknown metal.
The molar mass indicates that the metal must be titanium.
Example Question #1 : Reactions
Scandium can be plated out of a solution containing 
How many minutes would it take to plate out 

Recall the following formula:





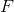
Start by finding how many moles of scandium was plated out.
Now, use the stoichiometric ratio present in the half reaction to find the number of moles of electrons.
Now, use the above formula to solve for the time.
Now, convert the seconds to minutes. Remember that your answer should only have 
All College Chemistry Resources