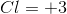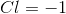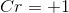All College Chemistry Resources
Example Questions
Example Question #2 : Reactions
Determine the oxidation number of each element in the compound
The oxidation number of each element in the compound 
The oxidation number of chlorine is 
Because this is a neutral atom, the overall charge is 0. Therefore we can set
Therefore,
We can then solve for the oxidation number for
Example Question #1 : Assigning Oxidation States
Given the following equation, identify the reducing agent.
Recall that a reducing agent is being oxidized and is losing electrons. Thus, when looking at the equation, look for which oxidation state is becoming more positive.
Start by assigning oxidation states to the elements.
For the reactants:







Now, assign the oxidation state of the products.








Only 
Example Question #1 : Assigning Oxidation States
In the given reaction, which element(s) is/are oxidized?


Let's start by looking at the equation and assigning oxidation numbers based off of the general rules we are given:
Let's start by looking at the reactants, namely 



Next let's look at 




so 

Now we have the oxidation numbers of all the elements present in this equation that are on the reactant side. I recommend writing down their oxidation numbers next to each other, so the elements that are oxidized and reduced can quickly be determined.
Oxidation numbers of elements in reactants:
Now let's look at the product side of the equation and determine the oxidation numbers of the elements there. First let's look at 







Finally, let's look 



Let's now write down the oxidation numbers of all the elements in the products.
Oxidation numbers of elements in products:
Now let's compare the oxidation numbers of the elements in the reactants with those in the products. 








Example Question #2 : Assigning Oxidation States
Which compound below has a nitrogen atom at a 
Let's start by going through each answer case-by-case applying the elementary rules of oxidation states we are given. The most applicable of these rules in this problem are the oxidation state of hydrogen in a compound is generally equal to 

Let's start by looking at 





solving for 



Let's still look at the other cases, however:
As this compound is uncharged we know it's net charge is equal to
Using our knowledge of oxidation states of hydrogen and oxygen and counting the number of hydrogens and oxygen in this compound, we can determine that the total charge of all the hydrogens together is equal to 

Just as we did above we can create an equation and solve for our unknown.
therefore 


Next let's look at 
We can quickly apply our knowledge of oxidation states of oxygen knowing that the oxidation state of oxygen in compounds is generally 



So our equation for this becomes:
therefore 

Finally the last compound is 
With this compound we can apply the rule we know about hydrogen's oxidation state in a compound being equal to 



Since there are two nitrogens, our equation is:
and 

Example Question #3 : Assigning Oxidation States
In which of the following compounds is the oxidation state of phosphorus the least?
Let's start by examining each of the compounds we are given case-by-case and applying the general rules of oxidation numbers we know.
Let's start with 
We know that based off of the general rules of oxidation states, Oxygen generally has an oxidation number of 



Now we can solve for the oxidation state of phosphorus:
Since we have 



This is equal to 

Solving for 


Next let's looking at 
Knowing that an atom in its elemental form has an oxidation state of 

Now let's look at 
Knowing that the oxidation number of hydrogen is 


so 

Now let's consider 
Using our elementary rules for the oxidation numbers, we know oxygen has a 



Therefore our equation is:
therefore 

Finally let's look at 
Using the rules stated above for 
Therefore 

This means that the compound with the lowest oxidation state is 
Example Question #4 : Assigning Oxidation States
What is the oxidation state of gold in 
Let's first consider the overall compound 






We can now solve for oxidation state of gold through creating an equation as shown below:
Simplifying this we get that 
Example Question #5 : Assigning Oxidation States
What species is reduced in the following chemical reaction?
Reduction is defined as the gain of electrons so we want to find the element that gains electrons/becomes negatively charged.
Once again our equation is
Let's start on the reactant side with 
Following our general rules of oxidation states, we know that an atom in its elemental form has an oxidation state equal to zero, therefore 

Next, consider 



We can now solve for the oxidation state of 


Now let's consider 
Applying the rule for the oxidation number of oxygen being 
where 
This simplifies so that 

Moving on to the products, let's consider 
We know that the oxidation state of Oxygen is 


Now we must solve for sulfur's oxidation state:
therefore 

As for 


Looking at the changes of oxidation numbers between elements in the products and reactants we see that there is no change in the oxidation state of sulfur, hydrogen, and oxygen between the products and reactants, but lead goes from 

Example Question #6 : Assigning Oxidation States
What is the oxidation state of copper in 
In order to determine the oxidation state of copper in this compound we first must note the fact that compound has an overall charge of 
We can also apply the general rules of oxidation numbers we know to determine the oxidation numbers of hydrogen and oxygen. Since hydrogen is a group 



As for oxygen, based off of our general rules of oxidation numbers, we know that oxygen normally has an oxidation number of 
therefore 

Example Question #7 : Assigning Oxidation States
What is the oxidation number of sulfur in 
In order to determine the oxidation number we must apply the general rules of oxidation numbers we know. One of these rules states the oxidation number of fluorine is always 



This equation is 



therefore the oxidation number of sulfur is 
Certified Tutor
Certified Tutor
All College Chemistry Resources