All ACT Science Resources
Example Questions
Example Question #54 : Chemistry
A student performed the following procedures to study various photosynthetic pigments (light-absorbing chemicals) in tree leaves and the wavelengths of light they absorb.
Experiment 1:
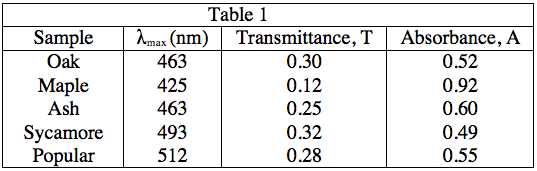
The student obtained samples of leaves from oaks, maples, ashes, sycamores, and poplars. Each leaf sample was ground separately with a mortar and pestle to release the pigments, and then each sample was suspended in water to make a colored solution of the pigment. The student then measured the absorption spectrum (a graph of how much light is absorbed by a pigment at varying wavelengths of light) of each solution in a device called a spectrophotometer. The setup of a spectrophotometer is shown below in Diagram 1.

The light source emits white light, which is split into its various wavelengths by the prism. Next, a slit, which can be moved up or down to select a particular wavelength, is used to transmit just a single wavelength to the sample. The sample absorbs a fraction of this light that is characteristic to the pigment in the sample, and the rest is transmitted to the detector for a readout. Using the spectrophotometer, the student found the λmax (the wavelength of light in nanometers (nm) that the pigment absorbs most intensely, for each sample) and recorded the results in Table 1. Table 1 also shows the transmittance and absorbance values at λmax. Transmittance, T, is defined as the fraction of light, expressed as a decimal, which passes through the sample. Absorbance, A, is given by:
A = –log(T) or 10–A = T
Experiment 2:
A student is given a leaf from an unknown source. She crushes and extracts the pigment according to the procedure in Experiment 1. Measuring the absorbance spectrum in the spectrophotometer produces the following readout, shown in Diagram 2.

Diagram 2
What is λmax, in nanometers, for the leaf in Experiment 2?
0.6
630
440
0.9
440
Experiment 1 states that λmax is the wavelength at which light is absorbed most intensely. Thus, we can look for the wavelength, found on the x-axis of Diagram 2, that produces the highest absorbance, found on the y-axis. This value is 440 nm, which produces an absorbance of 0.9.
Example Question #55 : How To Find Data Representation In Chemistry
A student wanted to study the kinetics, or rates of a chemical reaction based on the concentrations of its reactants and products, of the reaction shown below.

This reaction is easy to monitor using a spectrophotometer, which measures how much light of a particular wavelength is absorbed by a solution. The deep purple potassium permanganate, or 

Experiment 1:
The student constructed a standard curve, or a graph of the absorbance of solutions of varying concentrations of potassium permanganate, to quantify the relationship between concentration and absorbance. To prepare five sample of increasing concentration, he labeled five test tubes A, B, C, D, and E, weighed out 0.1, 0.2, 0.3, 0.4, and 0.5 grams of potassium permanganate into each, respectively, and added 1 milliliter (mL) of water to each test tube to dissolve. Then, he used the spectrophotometer to determine the absorbance at 550 nm of each sample. The data is graphed in Figure 1 below.

Figure 1
Experiment 2:
The student then studied potassium permanganate in the presence of oxalic acid, 

A sample solution of potassium permanganate in 1 milliliter of water was placed in a spectrophotometer and evaluated for its absorbance at 550 nm. It gave an absorbance of 0.3. How many grams of potassium permanganate were dissolved in the sample?
Figure 1 shows the relationship between absorbance and concentration of potassium permanganate. Look for the absorbance value of 0.3 on the vertical axis and see what concentration value on the horizontal axis would produce such an absorbance. The answer is 1.5 grams/mL. As the sample in this problem was dissolved in the same amount of water as in Experiment 1, we can assume that 0.15 grams were dissolved in this sample.
Example Question #56 : How To Find Data Representation In Chemistry
A student wanted to study the kinetics, or rates of a chemical reaction based on the concentrations of its reactants and products, of the reaction shown below.

This reaction is easy to monitor using a spectrophotometer, which measures how much light of a particular wavelength is absorbed by a solution. The deep purple potassium permanganate, or 

Experiment 1:
The student constructed a standard curve, or a graph of the absorbance of solutions of varying concentrations of potassium permanganate, to quantify the relationship between concentration and absorbance. To prepare five sample of increasing concentration, he labeled five test tubes A, B, C, D, and E, weighed out 0.1, 0.2, 0.3, 0.4, and 0.5 grams of potassium permanganate into each, respectively, and added 1 milliliter (mL) of water to each test tube to dissolve. Then, he used the spectrophotometer to determine the absorbance at 550 nm of each sample. The data is graphed in Figure 1 below.

Figure 1
Experiment 2:
The student then studied potassium permanganate in the presence of oxalic acid, 

Which of the following graphs could potentially generated if absorbance at 550 nm was graphed over time for a mixture of potassium permanganate and oxalic acid?

Blue graph
Green graph
Purple graph
Red graph
Red graph
As the absorbance is monitored at 550 nm, which will observe the behavior of potassium permanganate, a reactant, we know the absorbance should decrease as time goes on. The concentration of a reactant will decrease as it is used up by the reaction. Thus, the answer must be the red graph, as it is the only graph that shows consistently decreasing absorbance.
Example Question #741 : Act Science
Current high levels of fossil fuel use, including coal-burning power plants and gasoline-powered automobiles, have helped contribute to the high concentrations of sulfur trioxide, SO3, found in the atmosphere. When sulfur trioxide and water interact, they can undergo the following chemical reaction to produce sulfuric acid, which is the main contributor to acid rain worldwide:

Acid rain showers are particularly common near coal-burning power plants and large cities. These showers are responsible for significant economic damage to sidewalks, roads, and buildings. Scientists interested in studying the effects of acid rain often use basic substances like calcium carbonate, the main component of limestone buildings, and expose them to varying volumes of acid rain to determine what volume of acid rain is necessary to begin to erode a building. A sample graph of one scientist’s experiment is replicated below:

Measuring acid and base levels is commonly done with a scale called pH, which uses the concentration of hydrogen ions to determine the acidity. Hydrogen ions are in a balance with hydroxide ions to give a scale with a range from 0 to 14. Values equal to or between 0 and 6.9 represent the acidic range where hydrogen ions predominate and values equal to or ranging from 7.1 and 14 represent the basic range where hydroxide ions predominate. Thus, the more hydrogen ions present, the more acidic the solution.
Scientists can tell when a titration (pH) experiment passes a certain pH using compounds called indicators. Indicators are usually colorless at pH levels below that of their specified color change. A table of indicators used by the above scientists and the pH at which they change colors is presented below.

What is the pH of a solution containing calcium carbonate and sulfuric acid when 15 mL of sulfuric acid have been added?
7
10
2
5
10
This question asks us to use the provided figure to estimate the pH of a solution given an amount of acid added. Using the curve, we can see that a 15 mL addition of sulfuric acid corresponds to a pH of roughly 10. While we not be able to determine the exact number, we can use process of elimination with the answer choices to see which of the options presented is close to the number represented on the figure.
Example Question #741 : Act Science
The Ideal Gas Law is as follows:





A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.
|
Volume of the container |
Pressure Measured in Pascals |
|
1 cubic meter |
49,884 Pascals |
|
2 cubic meters |
24,942 Pascals |
|
3 cubic meters |
16,628 Pascals |
|
4 cubic meters |
12,471 Pascals |
|
5 cubic meters |
9,976.8 Pascals |
|
6 cubic meters |
8,314 Pascals |
|
7 cubic meters |
7,126.2 Pascals |
TABLE 1
And they graph their findings in Figure 1.

FIGURE 1
Will the pressure of a gas ever be 
Yes, when the volume of a gas is less than 0.5 cubic meters.
Yes. If the number of moles is 0 or if the temperature would ever reach 0 Kelvin.
No, because nothing is divisible by 0.
Yes, but only if the volume is 0 cubic meters.
No, because R will never be 0.
Yes. If the number of moles is 0 or if the temperature would ever reach 0 Kelvin.
The only way a gas can have a pressure of 
Example Question #742 : Act Science
The Ideal Gas Law is as follows:






A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.
|
Volume of the container |
Pressure Measured in Pascals |
|
1 cubic meter |
49,884 Pascals |
|
2 cubic meters |
24,942 Pascals |
|
3 cubic meters |
16,628 Pascals |
|
4 cubic meters |
12,471 Pascals |
|
5 cubic meters |
9,976.8 Pascals |
|
6 cubic meters |
8,314 Pascals |
|
7 cubic meters |
7,126.2 Pascals |
TABLE 1
And they graph their findings in Figure 1.

FIGURE 1
Will the graph ever cross the y-axis (the axis on which pressure is measured)?
Yes, but it will cross at less than 
Yes, when volume is 0 cubic meters.
Yes, when the controlled variable of the number of moles is changed to 40.
No, because in order for it to do that, Volume would have to be 0. Volume will never be 0 because an equation cannot be solved if it requires being divided by 0.
No, it would only cross the y-axis if the volume was infinite, and it is not possible to create a container with an inifinite number of cubic meters.
No, because in order for it to do that, Volume would have to be 0. Volume will never be 0 because an equation cannot be solved if it requires being divided by 0.
The lower the volume, the closer to the y-axis the graph will get, but it can never reach 0. If volume is measured at 0, the equation would have to be divided by 0. An equation cannot be divided by 0.
Example Question #743 : Act Science
The Ideal Gas Law is as follows:






A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.
|
Volume of the container |
Pressure Measured in Pascals |
|
1 cubic meter |
49,884 Pascals |
|
2 cubic meters |
24,942 Pascals |
|
3 cubic meters |
16,628 Pascals |
|
4 cubic meters |
12,471 Pascals |
|
5 cubic meters |
9,976.8 Pascals |
|
6 cubic meters |
8,314 Pascals |
|
7 cubic meters |
7,126.2 Pascals |
TABLE 1
And they graph their findings in Figure 1.

FIGURE 1
What is the pressure at temperature 0 Kelvin?
The answer cannot be determined from the data available.
The temperature and pressure are directly proportional to one another. If the temperature reaches 0 Kelvin (which is absolute 0, the temperature at which molecules no longer move at all), there will be 
Example Question #744 : Act Science
The rate of a reversible chemical reaction depends on many factors, including concentrations of the reactants and products, temperature, and presence of enzymes called catalysts. In the forward reaction, two reactants combine to form one product. However, in a reverse reaction, the product is broken down into the two reactants.
In order for a forward reaction to occur, the reactants moving around in the test tube must physically interact with each other. The more often reactants interact with each other, the more produce is formed in the same amount of time. The speed at which reactants combine into products (the rate of the reaction) can be calculated by dividing the amount of a chemical produced in a reaction (often measured in moles) by the time it takes to produce that amount.
In order to determine the effects of reactant and product concentration, temperature, and presence of catalysts on the rate of a reaction, a scientist studied the following reaction:
The scientist varied the conditions of the experiment and measured the rate of the reaction. The results are outlined in Table 1. The units of concentration are moles per liter.

In a further experiment, intead of using 5 or 10 moles for the amount of acid covertase, the scientist uses 20 moles. What is the expected rate of reaction?
120
80
160
40
160
Using Table 1 and Experiments 2 and 3, we can see that doubling the moles of acid convertase doubles the rate. Thus, using 20 moles instead of 10, we would expect to see a double in the rate of 80 to 160 moles per liter per second.
Example Question #745 : Act Science
The rate of a reversible chemical reaction depends on many factors, including concentrations of the reactants and products, temperature, and presence of enzymes called catalysts. In the forward reaction, two reactants combine to form one product. However, in a reverse reaction, the product is broken down into the two reactants. If the reverse reaction is running at a quick rate, the overall rate of product formation will be lowered.
In order for a forward reaction to occur, the reactants moving around in the test tube must physically interact with each other. The more often reactants interact with each other, the more produce is formed in the same amount of time. The speed at which reactants combine into products (the rate of the reaction) can be calculated by dividing the amount of a chemical produced in a reaction (often measured in moles) by the time it takes to produce that amount.
In order to determine the effects of reactant and product concentration, temperature, and presence of catalysts on the rate of a reaction, a scientist studied the following reaction:
The scientist varied the conditions of the experiment and measured the rate of the reaction. The results are outlined in Table 1. The units of concentration are moles per liter.

Why might doubling the number of moles of HCl decrease the rate of HCl production?
No more H+ or Cl- exists to be converted
The acid convertase enzyme has become inactive
The acid convertase enzyme is converting HCl back into H+ and Cl-
Cannot Be Determined
The acid convertase enzyme is converting HCl back into H+ and Cl-
The passage helps us understand that the purpose of an enzyme is to allow for the conversion of reactant to product and product back to reactant. According to Table 1, when the moles of HCl starting the reaction are doubled (Experiments 1 and 7), we see a 50% decrease in the rate of the reaction. It is reasonable that this decrease in the rate of HCl production is due to some of the HCl being driven back to H+ and Cl- according to information contained in the passage, where it says, "if the reverse reaction is running at a quick rate, the overall rate of product formation will be lowered."
Example Question #65 : Chemistry
The rate of a reversible chemical reaction depends on many factors, including concentrations of the reactants and products, temperature, and presence of enzymes called catalysts. In the forward reaction, two reactants combine to form one product. However, in a reverse reaction, the product is broken down into the two reactants.
In order for a forward reaction to occur, the reactants moving around in the test tube must physically interact with each other. The more often reactants interact with each other, the more produce is formed in the same amount of time. The speed at which reactants combine into products (the rate of the reaction) can be calculated by dividing the amount of a chemical produced in a reaction (often measured in moles) by the time it takes to produce that amount.
In order to determine the effects of reactant and product concentration, temperature, and presence of catalysts on the rate of a reaction, a scientist studied the following reaction:
The scientist varied the conditions of the experiment and measured the rate of the reaction. The results are outlined in Table 1. The units of concentration are moles per liter.

According to Table 1, how would halving the moles of H+ used change the rate of the reaction?
Decrease by 4 Times
Increase by Two Times
Increase by 4 Times
Decrease by Two Times
Decrease by Two Times
Comparing Experiments 1 and 4 in Table 1, we can see that halving the moles of H+ used in the reaction would drop the rate of the reaction by two times (i.e. halving it).
Certified Tutor
All ACT Science Resources





















