All ACT Science Resources
Example Questions
Example Question #48 : Chemistry
If a drug is taken orally, then the concentration of that drug in the blood will rise to a peak concentration. Immediately afterwards, the elimination phase begins and the concentration of the drug in the body will fall exponentially. Each drug has a half-life (i.e. the time required for the drug's concentration in the blood to fall to half of its original value). Once the elimination phase has initiated, it typically takes four half-lives until the drug has been entirely eliminated from the body.
If an individual is given multiple doses of a drug over time, then the blood concentration of the drug will rise and fall periodically. This creates a graph of drug’s concentration versus time that will resemble a wave. The rising peaks of the graph occur immediately after administration of a dose, whereas the falling valleys occur as the drug is being eliminated. The inter-dose interval, represented by the letter 
Experiment 1
A 125-milligram oral dose of a drug known as Cyclosporin A (CsA) was administered to an individual. The CsA blood concentration for this individual was then measured at various times over the next 24 hours. A graph of CsA concentration versus time was obtained.

Experiment 2
An individual was given multiple doses of CsA over a 60-hour period. The CsA blood concentration was monitored continuously. A graph of CsA concentration versus time was obtained.

Based on the results of Experiment 1 and the information in the passage, at which point does the elimination phase of CsA begin (assume the first data point is at zero hours)?
From hours 0 to 3 the concentration of CsA is rising. At 3 hours, it reaches a peak and begins to decline immediately afterwards; therefore, the elimination phase begins at 3 hours.
Example Question #49 : Chemistry
If a drug is taken orally, then the concentration of that drug in the blood will rise to a peak concentration. Immediately afterwards, the elimination phase begins and the concentration of the drug in the body will fall exponentially. Each drug has a half-life (i.e. the time required for the drug's concentration in the blood to fall to half of its original value). Once the elimination phase has initiated, it typically takes four half-lives until the drug has been entirely eliminated from the body.
If an individual is given multiple doses of a drug over time, then the blood concentration of the drug will rise and fall periodically. This creates a graph of drug’s concentration versus time that will resemble a wave. The rising peaks of the graph occur immediately after administration of a dose, whereas the falling valleys occur as the drug is being eliminated. The inter-dose interval, represented by the letter 
Experiment 1
A 125-milligram oral dose of a drug known as Cyclosporin A (CsA) was administered to an individual. The CsA blood concentration for this individual was then measured at various times over the next 24 hours. A graph of CsA concentration versus time was obtained.

Experiment 2
An individual was given multiple doses of CsA over a 60-hour period. The CsA blood concentration was monitored continuously. A graph of CsA concentration versus time was obtained.

Suppose drug administration had continued past 60 hours during Experiment 2. Based on the results of Experiment 2, what would be the most likely concentration of CsA at 66 hours (assume the drug continued to be administered in the same exact way)?
In order to answer this question, we can continue the pattern displayed in the graph of Experiment 2. Notice that a trough occurs at sixty hours. If we look at other troughs in the graph, then it becomes apparent that about six hours after these troughs the drug reaches the following concentration
As a result, the concentration at sixty-six hours would most likely be as follows:
Example Question #731 : Act Science
A chemist has found five substances in his lab without specific labels, named only A, B, C, D, and E. He hopes to find clues about the identity of each substance by dissolving each in hexane, a nonpolar solvent. He adds each substance to a different 
| Solute | Mass of Solute at Saturation (grams) |
| A | 11.1 |
| B | 23.6 |
| C | 0.23 |
| D | 3.1 |
| E | 0.11 |
Which substance has the highest solubility in hexane?
C
B
D
A
E
B

Example Question #51 : How To Find Data Representation In Chemistry

According to the graph at what temperature, in degrees celsius, are the solubilities for 

Between 70 and 80
Between 40 and 50
Between 60 and 70
Between 30 and 40
Between 10 and 20
Between 40 and 50
On the graph 

Example Question #52 : Chemistry

Based on the data in the graph, at 

Greater than
Less than
Between 
Greater than
Between 
Greater than
Assuming that the continuous positive slope of 


Example Question #52 : How To Find Data Representation In Chemistry
The table lists some of the properties of row 2 elements in the periodic table.

What conclusion can be drawn from the data in regards to atomic radius in row 2 elements on the periodic table?
An element with a low atomic radius will have a low atomic number
An element with a high atomic radius will have high electronegativity
An element with a low atomic radius will have low electonegativity
An element with a high atomic radius will be a metal
An element with a low atomic radius will be a metal
An element with a high atomic radius will be a metal
From studying the table it can be seen that the elements with the top two highest atomic radii are metals. As the atomic radius decreases elements are non-metals.
Example Question #54 : Chemistry
The table lists some of the properties of row 2 elements in the periodic table.

Which of the following graphs best represents the relationship between atomic radius and electronegativity for row 2 elements?






The table shows that as the atomic radius increases the electronegativity decreases. However, the atomic radius does not increase in continuous increments but in fact increases in larger subsequent increments as electronegativity decreases. Therefore, the graph will contain a curved line with a negative slope instead of a straight line.
Example Question #53 : Chemistry
Assuming that all of the weight lost or gained were solely from fat, determine the calories lost for the group subjected to only Inhibitor II. The energy density of fat is 9 calories per gram.
27,000 calories
22,000 calories
31,000 calories
14,000 calories
37,000 calories
27,000 calories
The approximate weight loss is about 3 kg for the Inhibitor II group. Therefore
Example Question #53 : How To Find Data Representation In Chemistry
Compute the mass of the fatty acid produced if inhibitor II were present in a sample of FAS and the experiment were to run for 20 seconds. Assume the fatty acid being produced is oleic acid, with a molecular weight of 282 grams.
.0014 grams
.0087 grams
.0056 grams
.0093 grams
.0021 grams
.0056 grams
Given that the rate of FAS with Inhibitor II present is 


Moles produced =
We can then compute the molecular weight by simply multiplying the number of moles by the molecular weight (282 grams), yielding 0.0056 grams.
Example Question #734 : Act Science
A student performed the following procedures to study various photosynthetic pigments (light-absorbing chemicals) in tree leaves and the wavelengths of light they absorb.
Experiment 1:
The student obtained samples of leaves from oaks, maples, ashes, sycamores, and poplars. Each leaf sample was ground separately with a mortar and pestle to release the pigments, and then each sample was suspended in water to make a colored solution of the pigment. The student then measured the absorption spectrum (a graph of how much light is absorbed by a pigment at varying wavelengths of light) of each solution in a device called a spectrophotometer. The setup of a spectrophotometer is shown below in Diagram 1.

The light source emits white light, which is split into its various wavelengths by the prism. Next, a slit, which can be moved up or down to select a particular wavelength, is used to transmit just a single wavelength to the sample. The sample absorbs a fraction of this light that is characteristic to the pigment in the sample, and the rest is transmitted to the detector for a readout. Using the spectrophotometer, the student found the λmax (the wavelength of light in nanometers (nm) that the pigment absorbs most intensely, for each sample) and recorded the results in Table 1. Table 1 also shows the transmittance and absorbance values at λmax. Transmittance, T, is defined as the fraction of light, expressed as a decimal, which passes through the sample. Absorbance, A, is given by:
A = –log(T) or 10–A = T
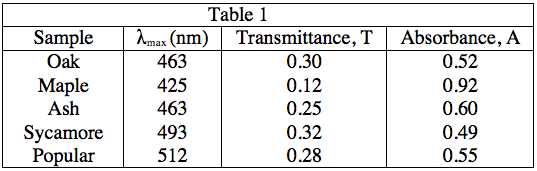
Experiment 2:
A student is given a leaf from an unknown source. She crushes and extracts the pigment according to the procedure in Experiment 1. Measuring the absorbance spectrum in the spectrophotometer produces the following readout, shown in Diagram 2.

Diagram 2
Which of the following leaves most likely have the same pigment in high quantities?
Oak and Sycamore
Maple and Sycamore
Maple and Ash
Oak and Ash
Oak and Ash
The description of Experiment 1 states that λmax is a value characteristic of a particular pigment. Because λmax = 436nm for both Oak and Ash leaves, it can be assumed that this is because both leaves contain large amounts of the same pigment.
Certified Tutor
All ACT Science Resources





















