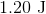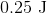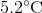All Physical Chemistry Resources
Example Questions
Example Question #11 : Thermochemistry And Thermodynamics
Argon gas is confined in a 



The work (


Plugging the values given into the work equation gives:
Example Question #12 : Thermochemistry And Thermodynamics
Argon gas is confined in a 



The work (


Plugging the values given into the work equation gives:
Example Question #1 : Isothermic, Isobaric, And Adiabatic Processes
Which of the following is not true for an isothermal process?
For an isothermal process there is no change in temperature, therefore, the temperature is a constant. An ideal gas has an internal energy, 
If you rearrange the equation, you will find that:
A simple explanation of this is that all the heat applied to the system is used to do the work. A piston is a good example for this phenomenon. This happens when every bit of energy applied or removed from a piston as work is done so slowly that all the heat has enough time to conduct into or out of the system and maintain a constant temperature.
Example Question #11 : Physical Chemistry
A sample of an ideal gas is initially at a volume of 



The expression for the relationship between heat (


The work (



Plugging work (
Plugging the values given into the internal energy equation gives:
Example Question #2 : Isothermic, Isobaric, And Adiabatic Processes
At 



The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there is no change in temperature. For an isothermal reversible process, the work done by the system is:
Plugging the values given into the equation gives:
Example Question #3 : Isothermic, Isobaric, And Adiabatic Processes
A sample of an ideal gas is initially at a volume of 



The expression for the relationship between heat (


The work (



Plugging work (
Plugging the values given into the internal energy equation gives:
Example Question #4 : Isothermic, Isobaric, And Adiabatic Processes
At 




The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there no change in temperature. For an isothermal reversible process, the work done by the system is:
Convert the grams to moles of argon:
Plugging the values given into the equation gives:
Example Question #1 : Isothermic, Isobaric, And Adiabatic Processes
Which of the following processes are considered an isothermic reaction?
I. Condensation
II. Evaporation
III. Sublimation
I, II and III
I only
I and II
II only
I, II and III
Isothermic reactions are characterized as reactions that occur in constant temperature. Recall that phase changes (such as condensation, evaporation, etc.) occur at a constant temperature (melting point, boiling point,e etc.). Consider the following example. Water boils at 

Example Question #7 : Isothermic, Isobaric, And Adiabatic Processes
Melting of a solid to a liquid is an example of what type of reaction?
Isobaric
Isothermic
More than one of these are true
Isovolemic
More than one of these are true
Isothermic reactions occur in constant temperature, isovolemic reactions occur in constant volume and isobaric reactions occur in constant pressure. During phase changes, like melting, the temperature stays constant (at the melting point). The pressure will also likely remain constant because most reactions are carried out at atmospheric pressure (
The volume, on the other hand, changes. Recall that gases have the highest volume, solids have the smallest volume and liquids have intermediate volume. This means that melting converts a low volume solid to higher volume liquid (therefore, this is not an isovolemic reaction). As a side note, recall that water is the only exception to this rule. Ice cube (solid water) has higher volume than liquid water.
Example Question #1 : Other Thermodynamic Principles
If a 

The thermal energy an object contains is given by:
Where 
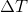
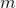


Plug in known values and solve:
Certified Tutor
All Physical Chemistry Resources