All Organic Chemistry Resources
Example Questions
Example Question #1 : Predicting Benzene Orientation
Predict the product of the given reaction.

IV
II
I
III
IV
This is an example of a Friedel-Crafts acylation reaction, where an acyl group (an alkyl group with a carbonyl 

Example Question #21 : Stereochemistry
What is the product of the reaction shown?

IV
I
II
III
II
This reaction is known as a nitration reaction, in which a nitro group (
Example Question #21 : Stereochemistry
Which of the following lists the product(s) of the presented reaction?

Methylcyclohexane
None of the other answers is correct.
Only trans-1-4-dimethylcyclohexane
Both trans and cis-1-4-dimethylcyclohexane
Only cis-1-4-dimethylcyclohexane
Both trans and cis-1-4-dimethylcyclohexane
Hydrogen and a catalyst like paladium reduce the double bond to a single bond. There is no equal steric hinderance on each side. The hydrogen can bond from either side. That means the methyl group can either be oriented into the page or out of the page. One form is cis, and one form is trans.
Example Question #2 : Isomers
I.
II.
III.
Which of the given molecules is(are) chiral?
I, II, and III
II and III
I and II
I only
I and III
I and III
For a molecule to be chiral, it must have a stereocenter and no axis of symmetry. An atom with a stereocenter has no identical bonds; it is a carbon atom with four unique substituents. There are two stereocenters in each of the three molecules. Notice that if you take the second molecule and draw a line connecting the top carbon and the point between the the two carbons with hydroxy groups, it has an axis of symmetry and therefore cannot be chiral. There is no way to draw that axis of symmetry for molecules one and three.
Example Question #21 : Stereochemistry

How many stereocenters does the given molecule have?
Two
Five
One
Three
Four
Two
A stereocenter exists when the central atom is bound to four unique substituents. In the given molecule, the carbons are numbers from left to right. Carbons 1, 3, 5, 6, and 8 are all bound to at least two hydrogen atoms and cannot be stereocenters. Similarly, the carbons in the two methyl groups (bound to C4 and C7) do not qualify as stereocenters. Carbon 7 has two identical methyl substituents. This leaves only C2 and C4. The molecule has two stereocenters.
Example Question #1 : Help With Cis Trans Isomers
What is the IUPAC name of the molecule shown?

E-5-carboxy-2-pentene
E-pent-3-enoic acid
Z-pent-3-enoic acid
Z-5-carboxy-2-pentene
Z-pent-3-enoic acid
Carboxylic acid is highest priority, so carbon chain labelled from right to left. Since highest priority groups are on the same side of the double bond, it's given the "Z" designation.
Example Question #1 : Help With Enantiomers
How many stereoisomers would be obtained by the hydrogenation of compound C?

Four
One
Two
Three
Zero
Two
The hydrogenation of compound C would add two hydrogen atoms across the double bond, but would generate only one new stereocenter. This stereocenter would be found on the third carbon in the chain (from the right), which would be bound to the phenyl substituent, a methyl group, a hydrogen atom, and the remaining branched carbon chain.
The hydrogenation reaction would create a racemic mixture of both possible orientations of this stereocenter, with both enantiomers present in equal amounts. There would this be two stereoisomer products obtained from the hydrogenation of compound C.
Example Question #2 : Isomers
How many stereoisomers are possible for the compound 2,3,4-trimethylpentane?
Three
Four
One
Two
Five
One
2,3,4-trimethylpentane does not contain any stereocenters. The structure is a five-carbon chain, with the end carbons bonded to three hydrogens each. The three central carbons each carry a methyl group and a hydrogen atom.
Remember that a stereocenter is only present when a carbon is bound to four different substituents. The 2 and 4 carbons both have two methyl groups (the end carbons and the added groups), so they would contain a plane of symmetry and would not be stereocenters. Likewise, a plane of symmetry exists at the 3 carbon; the substituents toward the 1 carbon and 5 carbon are the same. This compound, therefore, would have no stereocenters and could only exist as one stereoisomer.
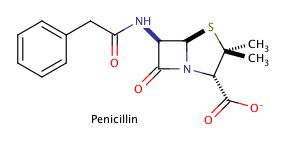
Example Question #22 : Stereochemistry
Shown above is the chemical structure for penicillin, a common prescription drug. How many chiral carbons does penicillin have?
Three
Zero
One
Two
Five
Three
The correct answer is three. The key to finding chiral carbons is to look for carbons that are attached to four different substituents. We can immediately eliminate any carbons that are involved in double bonds, or that have two hydrogens attached. Given this, we find that there are three chiral carbons. Note that carbon chains of varying content will qualify as different substituents, allowing chiral carbons to bond to two other carbons.
Example Question #4 : Isomers

Which two of the molecules shown are enantiomers?
III and IV
II and IV
I and III
II and III
I and II
I and III
The enantiomer of a molecule with multiple chiral centers is formed through configurational inversion at every chiral center. By rotating the ring of molecule III 180 degrees about the bond connected to its carbon chain, it is seen that molecules I and III are constitutionally identical with opposite configurations at every chiral center. These compounds are enantiomers. Tip: mental visualization of bond rotations and other transformations is among the most common difficulties experienced in organic chemistry courses. The use of a molecular modeling kit may greatly assist in molecular visualization. Molecules I and II are the same, just rotated 180 degrees.
Certified Tutor
Certified Tutor
All Organic Chemistry Resources