All Organic Chemistry Resources
Example Questions
Example Question #11 : Stereochemistry
What is the product of the reaction shown?

I only
III only
I and III
I, II, and III
I and III
In the molecule shown, the aldehyde group will direct incoming substituents to positions meta to it, and both the the hydroxyl group AND the fluorine group will direct incoming substituents to positions ortho or para to them. Options I and III show the incoming substituent meta to the aldehyde group, as well as either ortho or para to both the fluorine group and hydroxyl group. Therefore, they are both possible products.
Note: because option III shows attachment at a carbon that is slightly less sterically hindered than that of option I, option III will be produced in a slightly greater quantity.
Example Question #12 : Stereochemistry
Predict the major product in the reaction shown.

IV
II
I
III
III
The reagents shown will add a bromine to an aromatic ring through an electrophilic aromatic substitution mechanism. A nitro 

Example Question #13 : Assigning Ortho, Meta, Para
What is a major product of the following reaction?

I and II
I only
II only
III only
I only
This is an example of a Friedel-Crafts acylation reaction, which will add an acyl group (alkyl group containing a carbonyl (

This reaction proceeds using an electrophilic aromatic substitution reaction mechanism. The presence of the hydroxyl group on the benzene will affect where the incoming acyl group will attach. A hydroxyl group is a strong benzene activator, which will direct incoming substituents to positions ortho 

Example Question #13 : Stereochemistry
Predict the major product of the reaction shown.

III only
I and III
I only
II only
III only
This reaction proceeds using an electrophilic aromatic substitution reaction mechanism. The presence of the amino group on the benzene will affect where the incoming acyl group will attach. An amino group is a strong benzene activator, which will direct incoming substituents to positions ortho 

Example Question #15 : Assigning Ortho, Meta, Para
If the molecule phenol (hydroxybenzene) were to undergo electrophilic aromatic substitution, which carbon(s) will the hydroxyl group direct incoming substituents to? (Start labeling carbons with number 










Several resonance structures can be drawn for the molecule phenol. These are shown below.

Because the overall charge distribution puts partial negative charges on carbons 





Example Question #361 : Organic Chemistry
If the molecule nitrobenzene were to undergo an electrophilic aromatic substitution, on which carbon(s) will the incoming substituent likely be directed to? (When numbering carbons on the benzene ring, label the carbon containing the nitro group as carbon number 




All carbons are equally likely to be substituted.


Several resonance structures can be drawn for the molecule nitrobenzene. These are shown below.

From these resonance structures, an overall molecular electronic distribution can be determined:

Because the overall charge distribution puts partial positive charges on carbons 




Example Question #11 : Stereochemistry
What is the arrangement of the substituents of the major product when bromine in iron (III) bromide reacts with bromobenzene?
None of these
Ortho
Substituents will be on the same carbon
Para
Meta
Para
When speaking of the arrangemts of two substituents on a benzene, their positions can be ortho, meta, or para. Ortho substituents are bound to adjacent carbons (C1 and C2). Meta substituents are bound to carbons with one intermediary carbon (C1 and C3). Para substituents are opposite one another across the benzene ring (C1 and C4).
The bromines in the given reaction result in a para product because bromine is ortho/para directing. There is a significant amount of ortho product, but because of steric hinderance, the major product is para.
Example Question #1 : Predicting Benzene Orientation

Suppose that toluene, whose chemical structure is shown, is reacted with 




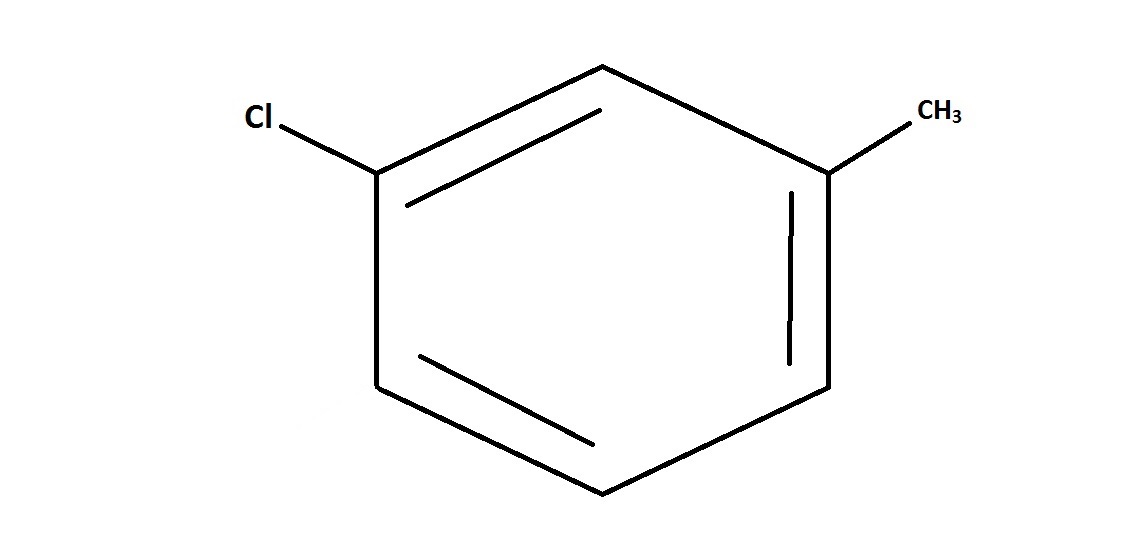

This question is essentially testing an understanding of electrophilic aromatic substitution and substituent placement. 


But we also need to be aware of what position this substituent will be directed to. Since the original molecule, toluene, consists of a benzene ring bonded to an electron-donating methyl group, this directs the substitution reaction towards the ortho or para position on the ring. Of the choices shown, only the para position is shown. The ortho position is not shown as an answer choice, and direction to the meta position would not be likely.
Example Question #13 : Benzene Additions

What is the product of the given reaction?






This is a trick question on Friedal-Craft alkylation. If you didn't pick the starting material, that is because you forgot the rule that alkylation cannot happen on a highly deactivated aromatic ring. Since 

Example Question #14 : Benzene Additions
What is the product for the following reaction?







While both the bromide and the alkyl group are ortho/para directing groups, the alkyl group is more activating and therefore the carbonyl will be added ortho to that substituent. The 


Certified Tutor
Certified Tutor
All Organic Chemistry Resources










