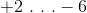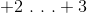All Organic Chemistry Resources
Example Questions
Example Question #1 : Finding Oxidation Number
The oxidation numbers of calcium and chromium in the compound 
Calcium is in the second group of the periodic table, so its oxidation number will always be 
As for chromium, the dichromate anion (






Example Question #1 : Finding Oxidation Number
What is the oxidation number of the manganese in potassium permanganate?
None of these
Manganese is a transition metal, so its oxidation number is more variable than the other elements of the compound. Oxygen is almost always 


The compound is neutral, so the manganese is 
Example Question #3 : Oxidation Reduction Reactions
Compared to oxygen in water, the oxygen in hydrogen peroxide has __________ valence electron(s).
Two more
One less
The same
One more
One less
To solve this question we need to calculate the oxidation number of oxygen in both molecules. The formula for water is 




Recall that have a negative charge suggests that an atom has extra valence electrons. A charge of 


Example Question #4 : Finding Oxidation Number
Which of the following is true regarding the correct oxidation number of potassium in potassium bromide?
Potassium bromide has a formula of 





Example Question #1 : Oxidation Reduction Reactions
Which of the following is the correct oxidation number of manganese in Manganese(III) oxide?
Manganese(III) oxide has a formula of 






Example Question #6 : Finding Oxidation Number
A student reacts sodium chloride and lithium bromide. He collects the products in a jar and performs several tests on them. He concludes that product A has a metal and a nonmetal and that the metal has an oxidation number of 

The identity of product A is lithium chloride
The identity of product A is sodium bromide
The other product will have similar oxidation numbers (metal: 

The results seem invalid
The results seem invalid
The reaction stated in this question is as follows.
This is a double replacement reaction. Both products contain a metal (an alkali metal) and a nonmetal (a halogen). The oxidation number of all alkali metals (first column of periodic table) is 


Example Question #7 : Finding Oxidation Number
In the compound 

Generally, oxygen has an oxidation number of 
















Certified Tutor
All Organic Chemistry Resources