All MCAT Biology Resources
Example Questions
Example Question #361 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The deprotonation of carbonic acid is favored by __________.
resonance stabilization in carbon dioxide
a loss of CO2 from the system
resonance stabilization in carbonic acid
a buildup of bicarbonate in the system
resonance stabilization in bicarbonate
resonance stabilization in bicarbonate
Bicarbonate is the product of deprotonation of carbonic acid. Anything that stabilizes this product will encourage the deprotonation reaction, and resonance is a key stabilizing factor for bicarbonate. Stability of the conjugate base is a major contributing factor to the strength of an acid and its ability to deprotonate.
Example Question #362 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
If a mutation rendered carbonic anhydrase nonfunctional, CO2 would not be converted to carbonic acid. What is true of CO2?
It has a greater dipole moment than carbonic acid
It has free rotation around the bonds of carbon and oxygen
It is more soluble in blood than carbonic acid
It has four sigma bonds
It is more soluble in membranes than carbonic acid
It is more soluble in membranes than carbonic acid
CO2 is nonpolar, and thus is more soluble in a nonpolar solvent (like a membrane) than is a polar molecule (like carbonic acid).
The pi bonds between carbon and oxygen in carbon dioxide contribute to its nonpolar character, and prevent free rotation around the bonds.
Example Question #363 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The carbonic acid buffer system is typical of most buffers. Which of the following is true of buffers?
They function best within one pH unit of target pH, and are used by combining equal and small amounts of an acid and its conjugate base
They function best within two pH units of target pH, and are used by combining equal and copious amounts of an acid and its conjugate base
They function best within two pH units of target pH, and are used by combining equal and copious amounts of an acid and an unrelated base
They function best within one pH unit of target pH, and are used by combining copious amounts of an acid and its conjugate base
They function best within two pH units of target pH, and are used by combining equal and small amounts of an acid and its conjugate base
They function best within one pH unit of target pH, and are used by combining copious amounts of an acid and its conjugate base
Buffers are typically acids best at keeping a system at a pH within one pH unit of their pKa when combined with equal and copious amounts of their conjugate base.
Example Question #364 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
Which of the following is true of carbonic acid?
I. It is diprotic
II. It is planar
III. It shows no net dipole moment
IV. It has approximately 109o bond angles
II, only
I, II, and III
I, II, and IV
I and II
I, II, III, and IV
I and II
Carbonic acid has a central sp2 carbon; thus, it is planar with approximately 120o bond angles. This carbonyl group also exhibits a strong dipole moment, and has hydrogens on both sides of the central carbon to donate in solution.
Example Question #16 : Functional Groups And Properties
Which of the following functional groups would most likely act as an acid?
Ketone
Carboxyl
Acetal
Aldehyde
Carboxyl
Carboxyl groups, or carboxylic acids, are good acids due to the resonance between the two oxygen atoms, allowing for greater stability of the conjugate base upon removal of a proton. Acetals and aldehydes can act as weak acids, but carboxyl groups will be deprotonated first.
Example Question #1751 : Mcat Biological Sciences
Carboxylic acids typically have higher boiling points than aldehydes and ketones. This is because carboxylic acids have which of the following properties?
Carboxylic acids can create intramolecular hydrogen bonds, which increases the boiling point
Aldehydes and ketones create stronger hydrogen bonds with water
Carboxylic acids can create intermolecular hydrogen bonds, increasing the effective molecular weight of the molecules
Carboxylic acids are less soluble in water than aldehydes and ketones
Carboxylic acids can create intermolecular hydrogen bonds, increasing the effective molecular weight of the molecules
Carboxylic acids are able to create hydrogen bonds with one another. This forms a dimer, which doubles the effective molecular weight and greatly increases the boiling point of carboxylic acids. Aldehydes and ketones are not able to form hydrogen bonds with one another, so their boiling points are dependent on each individual molecule's molecular weight. As a result, their boiling points are not as high as the corresponding carboxylic acids'. Note that carboxylic acids cannot form intramolecular hydrogen bonds.
Example Question #81 : Molecular Properties
A pentane molecule with which of the following functional groups will have a higher boiling point than an aldehyde of similar size?
Alkane
Alcohol
Ester
Alkene
Alcohol
Boiling point is strongly influenced by the ability of a molecule to interact with other molecules in solution (intermolecular forces). Since hydrogen bonds are the strongest intermolecular force, we are ideally looking for a functional group that will be able to form hydrogen bonds.
Alkanes and alkenes are both nonpolar and cannot form hydrogen bonds, so their boiling points are very low. Esters lack a polar hydrogen and cannot hydrogen bond with other molecules.
Alcohols, on the other hand, have a hydroxyl group that is capable of hydrogen bonding with other molecules. This gives alcohols higher boiling points than aldehydes of similar size.
Example Question #367 : Organic Chemistry, Biochemistry, And Metabolism
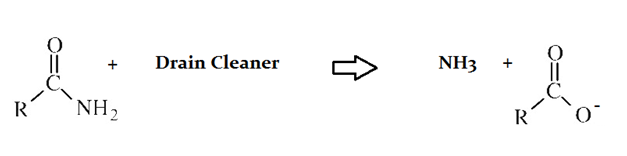
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Compared to the organic compound produced in Reaction 2, an aldehyde __________.
has no carbonyl group
has more polarity in its bonds
is more oxidized
has lower acidity
has lower overall bond energy
has lower acidity
Aldehydes have lower acidity than carboxylic acids, but are more reduced and thus have higher overall bond energies.
Example Question #1752 : Mcat Biological Sciences
In the reaction scheme below, compound A is a(n) __________ and compound B is a(n) __________.

alkene . . . alcohol
ketone . . . alkene
ketone . . . alcohol
alcohol . . . alkene
alcohol . . . ketone
ketone . . . alcohol
Ketones, like compound A, contain an internal carbon-oxygen double bond. Alcohols, like compound B, contain a hydroxyl group (-OH). In this case, compound A is a secondary ketone and compound B is a tertiary alcohol.
Alkenes, like compound C, contain a carbon-carbon double bond.
Example Question #363 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following compounds will be the most reactive with an alcohol to form an ester product?
Acid anhydride
Carboxylic acid
Acid chloride
Amide
Acid chloride
All of the options, except for the carboxylic acid itself, are derivatives of a carboxylic acid. These derivatives differ in their reactivity when creating new compounds. Acid chlorides are the most reactive compounds, and amides are the least reactive out of these option. Acid chlorides and acid anhydrides frequently participate in reactions, while amides are less likely to react.
Certified Tutor
Certified Tutor
All MCAT Biology Resources