All MCAT Biology Resources
Example Questions
Example Question #4 : Properties Of Lipids
Sexually transmitted diseases are a common problem among young people in the United States. One of the more common diseases is caused by the bacterium Neisseria gonorrhoeae, which leads to inflammation and purulent discharge in the male and female reproductive tracts.
The bacterium has a number of systems to evade host defenses. Upon infection, it uses pili to adhere to host epithelium. The bacterium also uses an enzyme, gonococcal sialyltransferase, to transfer a sialyic acid residue to a gonococcal surface lipooligosaccharide (LOS). A depiction of this can be seen in Figure 1. The sialyic acid residue mimics the protective capsule found on other bacterial species.
Once infection is established, Neisseria preferentially infects columnar epithelial cells in the female reproductive tract, and leads to a loss of cilia on these cells. Damage to the reproductive tract can result in pelvic inflammatory disease, which can complicate pregnancies later in the life of the woman.

What is likely true of the lipid A found on the glucosamine molecule in Figure 1?
It is composed predominately of hydrogens and carbons
It contains only nitrogen, carbon, and hydrogen
It is unable to anchor in the cell membrane due to its hydrophilicity
It is derived from host proteins
It is synthesized by ribosomes
It is composed predominately of hydrogens and carbons
Lipids are hydrocarbons, and are the most energy-rich biological macromolecules due to their heavily reduced state. This reduced state is a function of the roughly equal electronegativity between carbon and hydrogen atoms.
Example Question #272 : Organic Chemistry, Biochemistry, And Metabolism
Water is the solvent in which all chemical reactions take place for living organisms. In addition, water has a number of critical characteristics that allows it to be invaluable to life as we know it.
Which of the following statements is FALSE concerning the properties of water?
Water solvates hydrophobic molecules, such as fatty acids, and separates them.
Water plays a major role in the breakdown of macromolecules.
Water's strong surface tension allows certain bugs to "stand" on the water's surface.
Water's ability to form hydrogen bonds allow it to maintain a liquid state in nature.
Water solvates hydrophobic molecules, such as fatty acids, and separates them.
Water is able to solvate hydrophilic compounds. Hydrophobic molecules, such as fatty acids, are typically aggregated together by water and can be separated.
Example Question #11 : Lipids
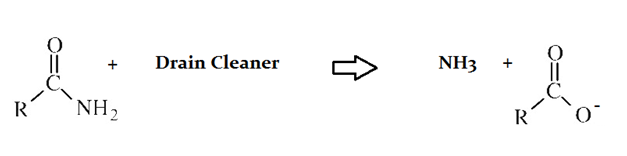
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The drain cleaner in Reaction 1 was used to break down fats as well as the protein depicted. How would the fats be most different in molecular structure?
They would be more reactive than the proteins.
They would be more oxidized than the proteins.
They would totally lack any polar bonds.
They would be more acidic than the proteins.
They would have much lower overall polarity in their bonds.
They would have much lower overall polarity in their bonds.
Fats are less reactive, more reduced, and less acidic than proteins. They have much lower overall polarity, but do contain polar bonds, such as between C and O.
Example Question #1 : Properties Of Lipids
Water often acts as a reactant or solvent in biological reactions. Which of the following cellular components would not be sufficiently solvated in the body?
Lipids
Amino acids
Nucleotides
Carbohydrates
Lipids
A molecule is solvated when it is surrounded by water molecules, and separated from the other molecules in the body. This separation is possible because of charge or polarity present in the molecule, which causes it to be attractive to water.
Lipids have very low solubility in water due to their nonpolarity. As a result, we conclude that lipids would not be properly solvated in the body, and would instead be clustered together by water molecules.
Example Question #1671 : Mcat Biological Sciences
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In contrast to the proteins in Reactions 1 and 2, the body uses carbohydrates __________.
to store energy for use over many days
to function as a secondary energy source after fatty acids have been oxidized
to dissolve nonpolar solutes in blood
to create strucutral elements of cells
to store energy for immediate use
to store energy for immediate use
Carboydrates are the main energy currency of cells. They are typically burned first, before fats and proteins, to generate energy. Proteins, in contrast, are usually the functional biomolecules, serving structural and enzymatic roles.
Example Question #1672 : Mcat Biological Sciences
Which of the following accurately describes glucose?
Aldopentose
Ketohexose
Ketopentose
Aldohexose
Aldohexose
Glucose is composed of six carbons. When not in ring form, there is an aldehyde at the end of the molecule. As a result, glucose is an example of an aldohexose.
Example Question #1 : Types Of Carbohydrates
Which of the following carbohydrates is created in animals, but not in plants?
Starch
Amylose
Glycogen
Cellulose
Glycogen
Glucose is stored in animals cells in the form of glycogen. Plants store glucose as either starch or cellulose. Amylose is a specialized component of starch, and plays a key role in plant energy storage.
Plants do not form glycogen, similar to the way that humans cannot form (or break down) cellulose.
Example Question #111 : Macromolecules
Which of the following statements is true concerning glucose?

Maltose and cellulose differ by the type of linkage between the glucose monomers
The 6th carbon attacks the carbonyl carbon in order to create the cyclic structure of glucose
D-glucose has the highest chiral carbon's hydroxyl group pointing to the left
Maltose and cellulose differ by the type of linkage between the glucose monomers
Maltose and cellulose are both composed of glucose monomers that are combined in a 1,4 glycosidic linkage, however they differ by the type of 1,4 linkage used. Cellulose uses a beta 1,4 linkage, while maltose uses an alpha 1,4 linkage.
Example Question #1 : Properties Of Carbohydrates
Which carbon in a carbohydrate determines whether a human is capable of digesting it properly?
The anomeric carbon
The first chiral carbon
The carbonyl carbon
All chiral carbons affect its digestion capabilities
The first chiral carbon
Humans are only capable of digesting the "D" isomer of a carbohydrate. The carbon that determines whether a carbohydrate is "D" or "L" is the first chiral carbon in the sugar. If it points to the right, the sugar can be digested by humans.
Example Question #1671 : Mcat Biological Sciences
Which type of bond is created between carbohydrates and the sidechain amine of select asparagine residues in proteins?
N-glycosidic
O-glycosidic
Both are correct
Neither is correct
N-glycosidic
A glycosidic bond covalently joins a carbohydrate molecule to another molecule. An O-glycosidic bond is a covalent linkage between a carbohydrate and a protein, joining a serine or threonine hydroxyl side chain and a sugar (oxygen in the bond yields "O"). An N-glycosidic linkage involves bonding of a carbohydrate and a protein, joining an asparagine side chain amide and a sugar (nitrogen in the bond yields "N"). Thus, N-glycosidic is the correct answer.
All MCAT Biology Resources