All High School Chemistry Resources
Example Questions
Example Question #22 : Acid Base Chemistry
What volume of a 1.2M solution of hydrochloric acid is needed to neutralize 50mL of a 3M sodium hydroxide solution?
The equation to use here is:
Here, 



Example Question #22 : Acids And Bases
What kind of reaction is an acid-base neutralization reaction?
Oxidation-reduction
Addition (synthesis)
Decomposition
Double-replacement
Single-replacement
Double-replacement
Below is a generic acid-base neutralization reaction:
The products are always water, and a salt. This salt is produced from the resulting ions 





Example Question #23 : Acids And Bases
Which of the following is the definition for an Arrhenius acid?
None of these
A substance that increases the 
A proton acceptor
A proton donor
A substance that increases the 
A substance that increases the 
Arrhenius acid/base theory was created by Swedish chemist Svante Arrhenius, and is the oldest acid/base classification. According to his classification, acids are compounds that increase the concentration of 


Example Question #23 : Acids And Bases
What is the concentration of hydronium ions in a solution if the hydroxide ion concentration is 
For every acidic or basic solution, the product of the hydroxide ion concentration and the hydronium ion concentration will be equal to 
Since we are given the hydroxide ion concentration, we can determine the hydronium ion concentration using this equation.
Example Question #431 : High School Chemistry
A student has a 0.50M solution of acetic acid. She adds solid sodium acetate until the concentration of sodium acetate is 0.050M. What is the final pH of the solution?
The 

Acetic acid is a weak acid, and its dissociation reaction is written as:
Using an ICE table, we can find the pH of the solution when the sodium acetate is added:
I: There is initially a 0.50M concentration of acetic acid. Because sodium acetate will dissolve completely, there will be a 0.050M concentration of acetate ions in the solution.
C: As acetic acid dissociates, the concentrations of acetate ions and hydronium ions will increase by an unknown concentration 
E: By setting the equilibrium expression equal to the acid equilibrium constant, we can solve for the value of 
*Because the value for 
Keep in mind that 
Use this value in the equation for pH:
Example Question #31 : Acids And Bases
What property distinguishes a strong acid from a weak acid?
A weak acid will dissociate completely in water and have a low Ka value
A strong acid will dissociate completely in water and have a low Ka value
A strong acid will dissociate completely in water and have a high Ka value
There is no difference between a strong acid and a weak acid based on their properties in water
A weak acid will fail to dissociate completely in water and have a high Ka value
A strong acid will dissociate completely in water and have a high Ka value
The acid dissociation constant (Ka) is used to distinguish strong acids from weak acids. Strong acids have exceptionally high Ka values.
The Ka value is found by looking at the equilibrium constant for the dissociation of the acid. The higher the Ka, the more the acid dissociates. Thus, strong acids must dissociate more in water. In contrast, a weak acid is less likely to ionize and release a hydrogen ion, thus resulting in a less acidic solution.
This equation makes it clear that the more the acid converts from its original form to its ionzied, dissociated form, the higher the Ka value will be.
Example Question #1 : Using Acid Dissociation Constant (Ka)
Which of the following is what determines the strength of an acid?
Electronegativity values
Its physical state
The Kb
How many bonds the central atom makes
The Ka
The Ka
The Ka is the acid dissociation constant, and thus it is what determines how strong the acid is. Stronger acids dissociate to a greater extent and produce lower pH values.
Example Question #31 : Acid Base Chemistry
Determine the equilibrium concentration of 


The definition of 

Set up an "ICE" table:
Plug in values:
Use the quadratic formula to solve:
Example Question #35 : Acid Base Chemistry
Determine the equilibrium concentration of 


Set up an "ICE" table
Plug in values:
Use the quadratic formula:
Example Question #31 : Acid Base Chemistry
A 1M solution of a monoprotic acid has a pH of 4.6. What is the 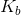
In order to find the base dissociation constant for the conjugate base, we can start by finding the acid dissociation constant for the acid. Since a 1M solution of the acid has a pH of 4.6, we can find the proton concentration of the solution.
Since the acid is monoprotic, we can set the following equilibrium expression equal to its acid dissociation constant.
We can see that, since the acid is monoprotic, the concntration of protons will be equal to the concentration of the acid anion. The final concentration of the acid molecule will be equal to the initial concentration, minus the amount of protons formed. Using these values, we can solve for the equilibrium constant for the acid.
Now that we have the acid dissociation constant, we can find the conjugate base's dissociation constant by setting the product of the two values equal to the autoionization of water.
All High School Chemistry Resources















![K_{sp}=[H_{3}O^{+}][OH^{-}] = 1*10^{-14}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/228880/gif.latex)
![[H_{3}O^{+}][OH^{-}] = 1*10^{-14}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/172798/gif.latex)
![[H_{3}O^{+}] = \frac{1*10^{-14}}{[OH^{-}]} = \frac{1*10^{-14}}{6.6*10^{-6}} = 1.5*10^{-9}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/172799/gif.latex)





![K_{a} = \frac{[C_{2}H_{3}O_{2}^-][H_{3}O^{+}]}{[HC_{2}H_{3}O_{2}]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/228970/gif.latex)
![K_{a} = \frac{[C_{2}H_{3}O_{2}^-][H_{3}O^{+}]}{[HC_{2}H_{3}O_{2}]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/228973/gif.latex)



![[H_3O^+]=1.8*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/228983/gif.latex)
![pH=-log[H_3O^+]=-log(1.8*10^{-4})=3.74](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/228984/gif.latex)

![K_a=\frac{[H^+][A^-]}{[HA]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/346504/gif.latex)

![[IO_3^-]=.00438M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583488/gif.latex)
![[IO_3^-]=.00420M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583492/gif.latex)
![[IO_3^-]=.00832M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/621410/gif.latex)
![[IO_3^-]=.00411M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583493/gif.latex)
![[IO_3^-]=.00377M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583490/gif.latex)
![K_a=0.16=\frac{[H^+][IO_3^-]}{[HIO_3]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/621414/gif.latex)

![K_a=.16=\frac{[X][X]}{[.0045-X]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583561/gif.latex)



![[IO_3^-]=.00438M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583930/gif.latex)

![[CH_3COO^-]=4.67*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/621717/gif.latex)
![[CH_3COO^-]=4.91*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584126/gif.latex)
![[CH_3COO^-]=8.11*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584127/gif.latex)
![[CH_3COO^-]=2.09*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584125/gif.latex)
![[CH_3COO^-]=6.55*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584128/gif.latex)
![K_a=1.76*10^{-5}=\frac{[H^+][CH_3COO^-]}{[CH_3COOH]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584355/gif.latex)

![K_a=1.76*10^{-5}=\frac{[X][X]}{[.0027-X]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584357/gif.latex)


![[CH_3COO^-]=2.09*10^{-4}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584360/gif.latex)


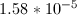

![pH=-\log[H^+]\rightarrow [H^+]=10^{-pH}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121717/gif.latex)
![\small [H^{+}] = 10^{-4.6} = 2.51*10^{-5}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94835/gif.latex)
![\small K_{a} = \frac{[H^{+}][A^{-}]}{[HA]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121718/gif.latex)
![K_a= \frac{[2.51*10^{-5}]^{2}}{[1-2.51*10^{-5}]} = 6.31*10^{-10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94836/gif.latex)