All High School Chemistry Resources
Example Questions
Example Question #5 : Calculating P H And P Oh
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
The pH of a solution of 
There are 100 times more protons in solution at pH 2 than at pH 4.
There are 100 times fewer protons in solution at pH 2 than at pH 4.
There are 20 times more hydroxide ions in solution at pH 2 than at pH 4.
There are 20 times fewer protons in solution at pH 2 than at pH 4.
There are 20 times more protons in solution at pH 2 than at pH 4.
There are 100 times more protons in solution at pH 2 than at pH 4.
The pH scale is logarithmic. Every pH unit drop corresponds to a tenfold increase in protons.
Example Question #9 : Calculating P H And P Oh
A sample of gastric juice has a pH of 2.5. What is the hydrogen ion concentration in this secretion?
The concentration of hydrogen ions must lie somewhere between 





To find the exact concentration, you must be familiar with the logarithmic scale. A difference of 0.5 is equivalent to a log of 3.
Our answer must therefore be 

We can calculate the pH in reverse to check our answer.
Example Question #1 : P H
What is the pOH of a 

The first step for this problem is to find the pH. We can then derive the pOH from the pH value.
The pH is given by the equation ![pH=-\log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96059/gif.latex)
Using this value and the pH equation, we can calculate the pH.
Now we can find the pOH. The sum of the pH and the pOH is always 14.
The pOH of the solution is 7.8.
Alternatively, a shortcut can be used to estimate the pH. If ![\small [H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/128818/gif.latex)


For this question, this shortcut gets us a pH of 6.4, which produces a pOH of 7.6; very close to the real answer!
Example Question #51 : Acids And Bases
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
A scientist is studying an aqueous sample of 

The pH of the solution is 3
The concentration of protons is
The pH of the solution is 11
The pOH of the solution is 11
The pH of the solution cannot be determined
The pH of the solution is 11
Given the hydroxide ion concentration, we will need to work using pOH to find the pH. We know that the sum of pH and pOH is equal to 14.
Use our value for the concentration to find the pOH.
Now that we have the pOH, we can use it to solve for the pH.
Example Question #63 : Acid Base Chemistry
An arterial blood sample from a patient has a pH of 7.4. One day later, the same patient has an arterial blood pH of 7.15. How many times more acidic is the patient's blood on the second day?
The equation to calculate pH is:
The normal pH of arterial blood is around 7.4. This reflects a concentration of hydrogen ions that can be found using the pH equation.
Using similar calculations for the second blood sample, we can find the hydrogen ion concentration again.
Now that we have both concentrations, can find the ratio of the acidity of the two samples.
You may know from biological sciences that this is approaching a lethal level of acidosis.
Example Question #61 : Acid Base Chemistry
You find a bottle in a lab that has a 
What is the pH of this solution?

Example Question #11 : Calculating P H And P Oh
Calculate the pOH and hydroxide ion concentration in an organism whose blood pH was measured to be 8.00 at 
To find the pOH note that 

We find the concentration of hydroxide ions based on the formula for pOH:
![[OH^-]=antilog(-pOH)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/470177/gif.latex)
Example Question #63 : Acid Base Chemistry
Calculate the pH of the following solution at 
Use the the dissociation constant for water 
Rearrange the dissociation constant to solve for the hydrogen ion concentration:
Plug in the given concentrations to find the actual hydrogen ion concentration:
Convert the hydrogen ion concentration to pH scale:
Example Question #61 : Acid Base Chemistry
Calculate the 


Relevant equations:

Combine equations:
Plug in values:
Example Question #1 : Concentration And Units
A 100mL solution is composed of 25% ethanol by volume and water. What is the mass of the solution?
First we determine the mass of the ethanol in solution using its density. Using the percent by volume of ethanol, we know that there are 25mL of ethanol in a 100mL solution. The remaining 75mL are water.
Since the density of water is 1g/mL, we know that the mass of 75mL of water is 75g. The total mass is the sum of the ethanol and the water.
All High School Chemistry Resources


![pH=-\log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/95534/gif.latex)







![pH=-\log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/129958/gif.latex)











![[HCl]=[H^+]=6*10^{-7}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/177010/gif.latex)








![pOH=-\log[OH^-]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/95553/gif.latex)









![\small \left [ H^{+}\right ]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/110187/gif.latex)
![\small 7.4 = - log\left [ H^{+}\right ]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/98016/gif.latex)
![\small 3.98 *10^{-8}M = \left [ H^{+}\right ]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/123319/gif.latex)
![7.15=-log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/98017/gif.latex)
![7.08*10^{-8}M=[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/110188/gif.latex)
![\frac{[H^+_2]}{[H^+_1]}=\frac{7.08*10^{-8}}{3.98*10^{-8}}=1.78](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/259310/gif.latex)

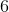




![[HNO_3]=[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/326585/gif.latex)
![pH=-log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/326586/gif.latex)


![[OH^-]=1.0*10^{-8}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471123/gif.latex)

![[OH^-]=1.0*10^{6}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471121/gif.latex)

![[OH^-]=8.0*10^{10}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471125/gif.latex)

![[OH^-]=6.0*10^{-10}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471119/gif.latex)

![[OH^-]=1.0*10^{-6}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471117/gif.latex)

![pOH=-log[OH^-]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/470176/gif.latex)
![[OH^-]=antilog(-6)=10^{-6}={1.0*10^{-6}M}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471128/gif.latex)






![K_{w}=[H^+][OH^-]=(1.0* 10^{-7})(1.0* 10^{-7})=1.0*10^{-14}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471085/gif.latex)
![[H^+]=\frac{K_{w}}{[OH^-]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/470145/gif.latex)
![[H^+]=\frac{1.0* 10^{-14}}{1.0* 10^{-5}}=1.0*10^{-9}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471086/gif.latex)
![pH=-log[H^+]=-log(1.0* 10^{-9}M)=9.00](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/471087/gif.latex)





![pH=-log([H^+])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583007/gif.latex)
![pOH=-log([OH^-])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583008/gif.latex)

![[H^+]=[HCl]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583012/gif.latex)
![pOH-log([H^+])=14](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/583014/gif.latex)
![pOH-log([.0750])=14](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/584906/gif.latex)












