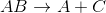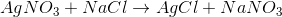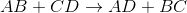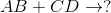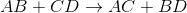All High School Chemistry Resources
Example Questions
Example Question #21 : Chemical Reactions
Consider the following balanced reaction:
When the reaction is at equilibrium, which of the following actions will result in more products?
Remove nitrogen gas
Increase temperature
Lower the temperature
Remove hydrogen gas
Lower the temperature
Le Chatelier's principle states that a system will shift in a particular direction in order to reduce introduced stress to the system. In other words, if something is added to one side of the equation, the other side will consequently increase in order to restore equilibrium.
The above reaction is exothermic, meaning that heat is released from the system. As a result, heat is considered a product.
By removing nitrogen or hydrogen gas, the system would shift to the left in order to create more reactants. If temperature is decreased, the system will create more heat by shifting to the right. Not only will this create more heat, but it will also create more ammonia, as they are both products of the reaction.
Example Question #22 : Chemical Reactions
Consider the following generic reaction:
If the equilibrium concentration of 

We can set up the equilibrium expression by placing the products, raised to the power of their coefficients, in the numerator and reactants, raised to the power of their coefficients, in the denominator:
We are given the value of the equilibrium constant and the equilibrium concentration of 




Simplify:
Now we can solve for 

Rounding to 2 sig figs (to match the two numbers we were given by the problem), we get:
Example Question #21 : Chemical Reactions
Which of the following reactions is an example of an addition reaction?
Addition reactions follow the general pattern:
When magnesium combines with oxygen gas, it results in a compound with both of them in it. This makes it a simple addition reaction. In general, addition reactions have a greater number of reactant compounds than product compounds.
The other answer choices correspond to the reactions below:
Decomposition reaction:
Single-replacement reaction:
Double-replacement reaction:
Example Question #1 : Types Of Reactions
What is another term for an addition reaction?
Single-replacement reaction
Hydrolysis reaction
Decomposition reaction
Double-replacement reaction
Synthesis reaction
Synthesis reaction
Addition reactions occur when two molecules combine to form a larger one, leading to a release of energy. Addition reactions result in the synthesis of a new molecule, giving them their second name: synthesis reactions.
Many synthesis reactions can also be classified as dehydration reactions, if water is formed as a byproduct. One example of this is the addition of amino acids to form protein chains. Hydrolysis reactions use water as a reactant and are used to split molecules apart (decomposition).
Example Question #2 : Types Of Reactions
What is another term for a dissociation reaction?
Dehydration reaction
Single-replacement reaction
Synthesis reaction
Double-replacement reaction
Decomposition reaction
Decomposition reaction
Dissociation reactions occur when one molecule is divided to form two smaller ones, leading to a decrease in energy. Dissociation reactions result in the break down of a large molecule to form smaller products, giving them their second name: decomposition reactions.
Many decomposition reactions can also be classified as hydrolysis reactions, if water is used as a reactant. One example of this is the breakdown of glycogen to form glucose monomers. Dehydration reactions release water in order to form a new bond, building a larger molecule from smaller reactants (synthesis).
Example Question #1 : Help With Dissociation Reactions
The reaction above could be classified as __________.
oxidation-reduction
single displacement
decomposition
decomposition and oxidation-reduction
synthesis
decomposition
When one molecule is converted into two or more smaller molecules, the reaction is considered a decomposition reaction. In this example, none of the elements under go a change in oxidation number, so this is not an oxidation-reduction reaction. (Calcium is always 


Example Question #1 : Help With Dissociation Reactions
In a dissociation reaction, determine the potential products for:
Dissociation reactions usually entail a salt or ionic compounds dissociating or splitting into its components. In this case, 
The options that include a third species, would be incorrect, as there are only two species in the original compound that is dissociating.
Example Question #2 : Types Of Reactions
Which of the following reactions involves both reduction and oxidation reactions?
The reaction of sodium chloride and calcium sulfate
Neither single displacement reactions nor the reaction of sodium chloride and calcium sulfate
Single displacement reactions
Both single displacement reactions and the reaction of sodium chloride and calcium sulfate
Single displacement reactions
A reaction that has both reduction and oxidation half reaction is called a redox reaction. It involves one or more atoms gaining electrons (reduction) and one or more atoms losing electrons (oxidation). Recall that single displacement reactions involve the replacement of an element in a compound with another element. An example of single replacement reaction is shown below:

In this reaction, a sodium atom replaces a calcium atom in calcium sulfate. If we calculate the oxidation numbers for sodium and calcium, we can see that sodium loses an electron (the oxidation state goes from 



Reaction of sodium chloride and calcium sulfate is as follows:
If we calculate the oxidation state of each atom we will notice that oxidation number doesn’t change; therefore, this isn’t a redox reaction.
Example Question #241 : High School Chemistry
What is the type of reaction seen below?
Addition
Double-replacement
Decomposition
Single-replacement
Double-replacement
By looking at the reaction, we see that the cations for the reactants have their anions switched in the products. This means that the reaction follows the general outline:
This is an example of a double-replacement reaction.
Addition reactions convert two reactants to a single product, while decomposition reactions convert a single reactant to multiple products. Single-replacement reactions only switch one cation-anion pair.
Example Question #1 : Help With Double Replacement Reactions
Predict the products for the following reaction if it were double displacement.
In a double displacement reaction, or double replacement reaction, opposite ions combine. What this means is that the cation of one compound will recombine and bond to the anion of the other compound and vice versa.
In this example, we have to remember the ideal convention of cations being written before the anion in the compound. So in AB, we can presume A being the cation and B being the anion. The same goes for CD - C is the cation and D is the anion.
With this in mind, we can now easily see how the replacement would be possible.
If A is a cation, and was originally bound to B (anion), the only other anion it is left with to bind is D. If C is a cation, and was originally bound to D (anion), it is only left with the option of binding to the now-free B anion.
That's why it is:
All High School Chemistry Resources












![K_{eq} = \frac{[A][B]^2}{[AB_2]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/217933/gif.latex)
![[A]=x,\ [B]=2x,\ [AB_2]=1](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/253911/gif.latex)



![\sqrt[3]{\frac{3.5*10^{-5}}{4}} = x](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/217937/gif.latex)