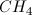All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #11 : Molecules And Compounds
Rank the following compounds in terms by increasing boiling point, starting with the lowest boiling point first.
I. 1-pentanol
II. n-pentane
III. 2,2-dimethylpropane
IV. (R)-4-hydroxypentanoic acid.
III < II < I < IV
IV < I < II < III
II < III < I < IV
II < III < IV < I
III < II < IV < I
III < II < I < IV
Boiling point is highly dependent on the intermolecular forces of a compound. Compounds with stronger intermolecular forces, larger masses, and less branching will have higher boiling points.
Compounds II and III only exhibit intermolecular London dispersion forces, so they would be the two lowest boiling compounds (weakest intermolecular forces). Because compound III has more branching, these London dispersion forces would be weaker, resulting in a lower boiling point than compound II.
III < II
Compounds I and IV would be higher boiling point compounds because of additional hydrogen bonding (strong intermolecular forces). Compound IV would be the highest boiling because the hydroxy group and carboxylic acid group could BOTH participate in intermolecular hydrogen bonding. In addition, compound IV is more polar (more polarized carbon-oxygen bonds), resulting in greater dipole-dipole attraction as well.
III < II < I < IV
Example Question #12 : Molecules And Compounds
Which molecule experiences only London dispersion forces, and no other intermolecular interactions?
Ammonia
Acetone
Liquid bromine
Ethanol
Liquid bromine
Intermolecular forces become relevant when there are partial charge differences between atoms in the molecule, generating polarized bonds. In order for this to happen, the two bonded atoms must have different electronegativities such that one atom pulls the electrons in the bond closer to it.
In liquid bromine , the two bromine atoms have the same electronegativity, so there is no unequal sharing of electrons. As a result, only London dispersion forces are found between bromine molecules.
Ethanol and ammonia are capable of hydrogen bonding, while acetone is capable of dipole-dipole interactions due to the polarized carbon-oxygen bond.
Example Question #3 : Other Intermolecular Forces
Which of the following solvents is the most polar?
![]()
Water is the most polar solvent. Polar molecules contain bonds that have charges that are opposite in charge with high electronegativity differences. The oxygen atom in water draws electrons away from the hydrogen atoms causing the oxygen to be more negatively charged and hydrogen atoms to be more positively charged. Water would not be polar without its bent geometry which allows it to have a non zero dipole moment (1.85D), making it a polar molecule. The polarity of water is essential for life as we know it.
Example Question #13 : Molecules And Compounds
Which solvent is miscible with water?
Cyclohexane
Pentane
Two solvents are miscible if after mixing them, they form a homogeneous mixture. The saying, like dissolves like, is an explanation of why benzene which is non polar will dissolve in a non polar solvent such as pentane. Polar molecules are soluble in polar solvents. For example, water is miscible in alcohols. Ionic substances such as common table salt which is composed of sodium chloride dissolve in water but do not dissolve to any great extent in most organic solvents which are non-polar. If we were to mix the benzene solution with water, we will find that they are not miscible and form a heterogenous mixture. Methanol , is the only polar solvent in the options given and is miscible with water.
Example Question #1 : Diagrams And Geometry
Which answer option correctly depicts the Lewis dot structure of sodium chloride?






When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
Sodium and chlorine form an ionic bond, meaning that one atom will donate an electron and the other will receive it. This gives each atom a charge. Chlorine has seven valence electrons, while sodium has one valence electron. For each atom to arrive at an octet, sodium will need to lose one electron and chlorine will need to gain one electron. This would give chlorine a negative charge, and sodium a positive charge.
Thus, the answer is a sodium with a positive charge (due to one lost electron) and a chlorine with eight electrons and a negative charge (due to one electron gained).
Example Question #51 : General Chemistry
What is the Lewis dot structure for ?






When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
In this case, boron actually has an incomplete octet. Though there are resonance forms in which boron has a full octet, when you calculate the formal charge of these configurations it will not be zero.
Example Question #51 : General Chemistry
Which of the following molecules will have the largest bond angle?
According to VSEPR theory, atoms will orient themselves in order to be as far away from neighboring atoms as possible in a molecule. This theory helps predict the geometry that molecules will take, as well as the bond angles between atoms.
is formed by a central carbon bound to two adjacent oxygen atoms. To maximize the distance between the oxygen atoms, they will align at an angle of 180 degrees, creating a linear shape.
All of the other given moleules will have bond angles less than 180 degrees.
Example Question #1 : Vsepr Geometry
Which of the following is not the correct geometric configuration for the given molecule?
, linear
, bent
, trigonal bipyramidal
, tetrahedral
, trigonal planar
, trigonal bipyramidal
Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #56 : Gre Subject Test: Chemistry
Which of the following molecules exhibits a trigonal pyramidal geometry?
The trigonal pyramidal geometry is implemented by molecules in which the central atom has three atoms and a lone pair attached. has three hydrogens attached to the central phosphorus as well as a lone pair, which can be determined by drawing the Lewis structure of the molecule. As a result, it has trigonal pyramidal geometry.
is trigonal planar. is tetrahedral. is octahedral.
Example Question #2 : Diagrams And Geometry
Which of the following molecules has an electronic geometry that is the same as its molecular geometry?
Electronic and molecular geometries are only the same when there are no lone pairs around the central atom in the molecule. is the only given option that does not have a lone pair on the central atom, so the electronic geometry is the same as the molecular geometry (in this case, tetrahedral).
Certified Tutor
Certified Tutor
All GRE Subject Test: Chemistry Resources